Impact of routine prophylaxis with monoclonal antibodies and maternal immunisation to prevent respiratory syncytial virus hospitalisations, Lombardy region, Italy, 2024/25 season
- PMID: 40211969
- PMCID: PMC11987492
- DOI: 10.2807/1560-7917.ES.2025.30.14.2400637
Impact of routine prophylaxis with monoclonal antibodies and maternal immunisation to prevent respiratory syncytial virus hospitalisations, Lombardy region, Italy, 2024/25 season
Abstract
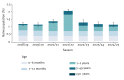
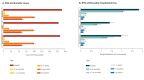
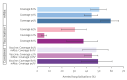
BackgroundRespiratory syncytial virus (RSV) is a leading cause of hospitalisation in children worldwide. Recent regulatory approval of monoclonal antibody (mAb) nirsevimab for infants and the RSVpreF vaccine for pregnant women offers promising approaches to mitigate RSV-associated morbidity.AimTo evaluate potential impacts of routine prophylactic campaigns (mAbs targeting infants or maternal vaccination) introduced in the 2024/25 season on hospitalisations from RSV lower respiratory tract infections in Lombardy, Italy.MethodsWe used a catalytic model informed by data from pre-COVID-19 pandemic (before 2020) and post-pandemic periods (until 2022) to quantify the number of cases and hospitalisations that could be averted by seasonal nirsevimab administration to infants and RSVpreF maternal vaccination, considering changes in susceptibility caused by reduced RSV circulation during the pandemic.ResultsAs a marked proportion of RSV hospitalisations occurs in infants aged ≤ 1 year, seasonal mAb administration to 80% of newborns (uptake levels observed in Spain) was estimated to avert 50.2% (95% CI: 43.5-55.8) of hospitalisations in the total population. Coverage levels close to those observed for childhood vaccines (95%) could result in an additional average 18% reduction in hospitalisations. Vaccination of 65% of pregnant women, resembling the diphtheria-tetanus-pertussis vaccine coverage in Lombardy for this population, was estimated to avert 30.5% (95% CI: 19.6-39.7) of hospitalisations. At influenza vaccine coverage (12%), less than 8% of hospitalisations could be averted by maternal immunisation.ConclusionRoutine nirsevimab administration to infants demonstrates clear potential to reduce RSV-associated hospitalisations. Maternal immunisation can help in achieving high protection in at-risk populations.
Keywords: immunization programs; maternally-acquired immunity; monoclonal antibodies; respiratory syncytial virus; respiratory syncytial virus vaccines; transmission model.
Conflict of interest statement
Figures
References
-
- Bénet T, Sánchez Picot V, Messaoudi M, Chou M, Eap T, Wang J, et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: The GABRIEL pneumonia multicenter, prospective, case-control study. Clin Infect Dis. 2017;65(4):604-12. 10.1093/cid/cix378 - DOI - PMC - PubMed
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047-64. 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- European Medicines Agency (EMA). New medicine to protect babies and infants from respiratory syncytial virus (RSV) infection. Amsterdam: EMA. [Accessed: 10 Jan 2024]. Available from: https://www.ema.europa.eu/en/news/new-medicine-protect-babies-and-infant...
-
- European Medicines Agency (EMA). First RSV vaccine to protect infants up to 6 months of age and older adults. Amsterdam: EMA. [Accessed: 20 Feb 2024]. Available from: https://www.ema.europa.eu/en/news/first-rsv-vaccine-protect-infants-6-mo...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
