Nanoparticle Targeting Strategies for Lipid and Polymer-Based Gene Delivery to Immune Cells In Vivo
- PMID: 40212067
- PMCID: PMC11935263
- DOI: 10.1002/smsc.202400248
Nanoparticle Targeting Strategies for Lipid and Polymer-Based Gene Delivery to Immune Cells In Vivo
Abstract
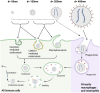
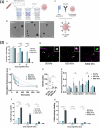
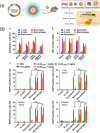
Lipid nanoparticles and polymeric nanoparticles are promising biomaterial platforms for robust intracellular DNA and mRNA delivery, highlighted by the widespread use of nanoparticle- (NP) based mRNA vaccines to help end the COVID-19 pandemic. Recent research has sought to adapt this nanotechnology to transfect and engineer immune cells in vivo. The immune system is an especially appealing target due to its involvement in many different diseases, and ex vivo-engineered immune cell therapies like chimeric antigen receptor (CAR) T therapy have already demonstrated remarkable clinical success in certain blood cancers. Although gene delivery can potentially address some of the cost and manufacturing concerns associated with current autologous immune cell therapies, transfecting immune cells in vivo is challenging. Not only is extrahepatic NP delivery to lymphoid organs difficult, but immune cells like T cells have demonstrated particular resistance to transfection. Despite these challenges, the modular nature of NPs allows researchers to examine critical structure-function relationships between a particle's properties and its ability to specifically engineer immune cells in vivo. Herein, several nanomaterial components are outlined, including targeting ligands, nucleic acid cargo, chemical properties, physical properties, and the route of administration to specifically target NPs to immune cells for optimal in vivo transfection.
Keywords: biomaterials; biotechnology; gene delivery; immunoengineering; nanotechnology.
© 2024 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
J.J.G. is a co‐founder, manager and CTO of Dome Therapeutics, co‐founder, board member and CSO of Cove Therapeutics, co‐founder of WyveRNA Therapeutics, and on the scientific advisory board of Mana.bio. These potential competing interests are managed by the Johns Hopkins committee on outside interests.
Figures





Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Co-assembly of oligo-urethane nanoparticles with defined lipid additives to tailor RNA delivery into cells.Acta Biomater. 2025 Jul 1;201:457-470. doi: 10.1016/j.actbio.2025.06.017. Epub 2025 Jun 14. Acta Biomater. 2025. PMID: 40523574
-
Nucleic Acid Nanocapsules as a New Platform to Deliver Therapeutic Nucleic Acids for Gene Regulation.Acc Chem Res. 2025 Jul 1;58(13):1951-1962. doi: 10.1021/acs.accounts.5c00126. Epub 2025 Jun 9. Acc Chem Res. 2025. PMID: 40491030
-
B cell antigens: A key to optimizing CAR-T cell therapy.Int Rev Immunol. 2025 Jun 19:1-28. doi: 10.1080/08830185.2025.2515839. Online ahead of print. Int Rev Immunol. 2025. PMID: 40537997 Review.
Cited by
-
Glyco Ionic Liquids as Novel Nanoparticle Coatings to Enhance Triple-Negative Breast Cancer Drug Delivery.Adv Healthc Mater. 2025 Jul 9:e2500592. doi: 10.1002/adhm.202500592. Online ahead of print. Adv Healthc Mater. 2025. PMID: 40635271
-
Enhancing nucleic acid delivery by the integration of artificial intelligence into lipid nanoparticle formulation.Front Med Technol. 2025 Jun 16;7:1591119. doi: 10.3389/fmedt.2025.1591119. eCollection 2025. Front Med Technol. 2025. PMID: 40589473 Free PMC article. Review.
-
Applications of nanoparticles in CAR-T cell therapy: non-viral manufacturing, enhancing in vivo function, and in vivo generation of CAR-T cells.Med Oncol. 2025 Jul 26;42(9):378. doi: 10.1007/s12032-025-02928-6. Med Oncol. 2025. PMID: 40715608 Review.
References
-
- FDA approval Brings First Gene Therapy to the United States | FDA 2017, https://www.fda.gov/news‐events/press‐announcements/fda‐approval‐brings‐... (accessed: April 2024).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
