Efficient Nebulization and Pulmonary Biodistribution of Polymeric Nanocarriers in an Acute Lung Injury Preclinical Model
- PMID: 40212072
- PMCID: PMC11935039
- DOI: 10.1002/smsc.202400066
Efficient Nebulization and Pulmonary Biodistribution of Polymeric Nanocarriers in an Acute Lung Injury Preclinical Model
Abstract
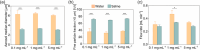
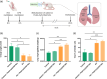
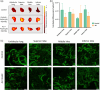
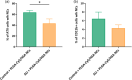
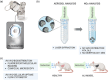
Acute respiratory distress syndrome (ARDS) is a clinical syndrome characterized by acute hypoxemic respiratory failure. Pneumonia and sepsis are the most common causes, turning ARDS into a critical public health problem. Despite recent advances in pharmacological strategies, clinical trials have not demonstrated a reduction in ARDS-associated mortality. This is in part connected to the singularity of the pulmonary physiological barrier, which hampers drug delivery, specifically at distal areas. To this aim, the use of polymeric nanocarriers as a platform for the efficient delivery of therapeutics to the lungs by nebulization is introduced. Herein, poly(lactic-co-glycolic acid) (PLGA) nanocapsules (NCs) loaded with human serum albumin, as an inhalable nanotherapeutic are prepared. The production of stable NCs aerosols in the inhalable range is achieved using a commercial device, while the nanocarrier's physicochemical parameters are only minimally altered after nebulization. Importantly, in vivo studies with healthy and acute lung injury animals show that after inhalation, the NCs are homogeneously distributed throughout the lungs, arriving at the distal areas. The NCs are internalized by alveolar type II cells, avoiding macrophage-mediated lung clearance. These features make the PLGA NCs excellent vehicles for noninvasive pulmonary delivery, facilitating a ready-to-be-used nanomedicine.
Keywords: inhalation; lung; nanocarriers; poly(lactic‐co‐glycolic acid); vibrating mesh nebulizer.
© 2024 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Matthay M. A., Arabi Y., Arroliga A. C., Bernard G., Bersten A. D., Brochard L. J., Calfee C. S., Combes A., Daniel B. M., Ferguson N. D., Gong M. N., Gotts J. E., Herridge M. S., Laffey J. G., Liu K. D., Machado F. R., Martin T. R., McAuley D. F., Mercat A., Moss M., Mularski R. A., Pesenti A., Qiu H., Ramakrishnan N., Ranieri V. M., Riviello E. D., Rubin E., Slutsky A. S., Thompson B. T., Twagirumugabe T., et al., Am. J. Respir. Crit. Care Med. 2023, 209, 37. - PMC - PubMed
-
- Grasselli G., Calfee C. S., Camporota L., Poole D., Amato M. B. P., Antonelli M., Arabi Y. M., Baroncelli F., Beitler J. R., Bellani G., Bellingan G., Blackwood B., Bos L. D. J., Brochard L., Brodie D., Burns K. E. A., Combes A., D’Arrigo S., De Backer D., Demoule A., Einav S., Fan E., Ferguson N. D., Frat J. P., Gattinoni L., Guérin C., Herridge M. S., Hodgson C., Hough C. L., Jaber S., et al., Intensive Care Med. 2023, 49, 727.
LinkOut - more resources
Full Text Sources
Other Literature Sources
