Fractal Characterization of Simulated Metal Nanocatalysts in 3D
- PMID: 40212231
- PMCID: PMC11935087
- DOI: 10.1002/smsc.202400123
Fractal Characterization of Simulated Metal Nanocatalysts in 3D
Abstract
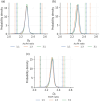
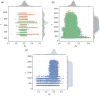
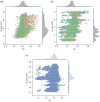
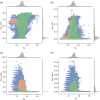
The surface roughness of metal nanoparticles is known to be influential toward their properties, but the quantification of surface roughness is challenging. Given the recent availability of large-scale simulated data and tools for the computation of the box-counting dimension of simulated atomistic objects, researchers are now enabled to study the connections between the surface roughness of metal nanoparticles and their properties. Herein, the relationships between the fractal box-counting dimension of metal nanoparticle surfaces and structural features relevant to experimental and computational studies are investigated, providing actionable insights for the manufacturing of rough nanoparticles. This approach differs from conventional concepts of roughness, but introduces a possible indicator for their functionalities such as catalytic performance that was not previously accessible. It is found that, while it remains difficult to consistently correlate the dimension with the catalytic activity of surface facets, matching trends with their surface energy, thermodynamic stability, and number of bond vacancy are observed. This highlights the potential of fractal box-counting dimensions to rationalize catalytic activity trends among metal nanoparticles, and opens up opportunities for the design of nanocatalysts with better performance via surface engineering.
Keywords: fractal dimension; metal nanoparticles; structural features; surface roughness.
© 2024 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Rodrigues T. S., da Silva A. G. M., Camargo P. H. C., J. Mater. Chem. A 2019, 7, 5857.
-
- Cui C.‐H., Yu S.‐H., Acc. Chem. Res. 2013, 46, 1427. - PubMed
-
- Jeon T. Y., Yu S. H., Yoo S. J., Park H. Y., Kim S. K., Carbon Energy 2021, 3, 375.
-
- Cui C.‐H., Li H.‐H., Liu X.‐J., Gao M.‐R., Yu S.‐H., ACS Catal. 2012, 2, 916.
LinkOut - more resources
Full Text Sources
