Xanomeline and Trospium Chloride Versus Placebo for the Treatment of Schizophrenia: A Post Hoc Analysis of Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed
- PMID: 40212458
- PMCID: PMC11981872
- DOI: 10.2147/NDT.S503494
Xanomeline and Trospium Chloride Versus Placebo for the Treatment of Schizophrenia: A Post Hoc Analysis of Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed
Abstract
Purpose: Describe xanomeline and trospium chloride efficacy and safety/tolerability for the treatment of schizophrenia using number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
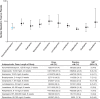
Methods: Categorical data were extracted from the three 5-week, randomized, double blind, placebo controlled EMERGENT-1, EMERGENT-2, and EMERGENT-3 clinical trials of xanomeline/trospium in adults with schizophrenia experiencing acute psychosis. Efficacy was assessed using the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impression-Severity (CGI-S), and categorical response criteria. Safety and tolerability were assessed using rates of discontinuation and treatment-emergent adverse events (TEAEs). NNT, NNH, and LHH values were calculated for each individual study as well as pooled.
Results: In data from the acute EMERGENT trials, NNT estimates were significant for xanomeline/trospium vs placebo for the pre-specified treatment response threshold of ≥30% reduction from baseline in PANSS total score at Week 5 (NNT=5 [95% CI, 4-8]). NNT estimates for response thresholds of ≥20% and ≥40% reduction from baseline in PANSS total score and ≥1- and ≥2-point decrease from baseline in CGI-S score were <10, indicating a clinically relevant therapeutic benefit of xanomeline/trospium over placebo. Estimates of NNH vs placebo for the most common TEAEs were >10, with the exception of nausea and vomiting; however, rates of discontinuations due to TEAEs of nausea, dyspepsia, or vomiting were low (NNH=49 [95% CI, 28-182]). LHH indicated an overall benefit of xanomeline/trospium vs placebo for all assessed outcomes. In indirect comparisons based on published data from trials of available antipsychotics approved for schizophrenia, xanomeline/trospium exhibited comparable or more robust NNT estimates vs placebo and was the least likely agent to be associated with weight gain or somnolence/sedation.
Conclusion: In the 5-week EMERGENT clinical trials, NNT, NNH, and LHH assessments demonstrated a favorable benefit-risk profile for xanomeline/trospium.
Trial registration: ClinicalTrials.gov identifiers: NCT03697252, NCT04659161, NCT04738123.
Keywords: KarXT; antipsychotic; number needed to harm; number needed to treat; schizophrenia; xanomeline and trospium chloride.
© 2025 Citrome et al.
Conflict of interest statement
Dr. Citrome has received payment from Karuna Therapeutics, a Bristol Myers Squibb company, for the design and execution of this analysis and has received other advising, consulting, and/or speaker fees from AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, Impel, INmune Bio, Intra-Cellular Therapies, Janssen, Karuna Therapeutics, a Bristol Myers Squibb company, Lundbeck, Luye, Lyndra, MapLight, Marvin, MedAvante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Takeda, Teva, University of Arizona, Vanda, and Wells Fargo, and performed one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research, CME activities organized by medical education companies such as HMP, Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies. He owns stocks from Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer and Reviva (options). Editor in Chief for Current Medical Research and Opinion, Taylor and Francis. Former Editor in Chief for International Journal of Clinical Practice, Wiley. Royalties from UpToDate, Springer Healthcare, and Elsevier. Dr. Neugebauer and Ms. Meli are employees of Bristol Myers Squibb. Dr. Kando is an employee of Bristol Myers Squibb and holds equity in Johnson and Johnson, McKesson, and Takeda Pharmaceuticals. The authors report no other conflicts of interest in this work.
Figures
References
-
- Kaul I, Sawchak S, Correll CU, et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet. 2024;403(10422):160–170. doi:10.1016/S0140-6736(23)02190-6 - DOI - PubMed
-
- NIH. A study to assess efficacy and safety of KarXT for the treatment of psychosis associated with Alzheimer’s disease (ADEPT-1). Available from: https://clinicaltrials.gov/study/NCT05511363. Accessed September 19, 2024.
Associated data
LinkOut - more resources
Full Text Sources
Medical