Strategies of Anode Design for Seawater Electrolysis: Recent Development and Future Perspective
- PMID: 40212636
- PMCID: PMC11935838
- DOI: 10.1002/smsc.202200030
Strategies of Anode Design for Seawater Electrolysis: Recent Development and Future Perspective
Abstract
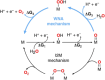
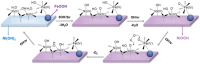
Compared with freshwater splitting, seawater electrolysis has more spaces to be explored, which is primarily attributed to the additional critical catalytic challenges of the competition between anodic oxygen evolution reaction (OER) and chlorine chemistry, deep corrosion, and site blocking due to the presence of chloride ions and insoluble particulate in seawater. However, if direct seawater electrolysis can be realized, it would revolutionize the energy and environmental sectors. In this review, the current effective strategies are summarized, including electronic modulation, oxygen vacancies creation, amorphous and porous structure design, corrosion-resistant passive layer decoration, and creating strong catalyst-support interactions. The review also provides insights for seawater electrolysis on rational design of the OER catalyst with high selectivity, activity, corrosion resistance, chemical, and mechanical durability. Beyond the progress made to date, a perspective in the fabrication of high-performance anodes for direct seawater electrolysis is also proposed. Collectively, this review will shed light on the rational design of a viable anode for massive and sustainable hydrogen fuel production from immense seawater.
Keywords: anode designs; electronic structures; hydrogen production; nanomaterials; seawater electrolysis.
© 2022 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Electronic and Structural Modification of Mn3O4 Nanosheets for Selective and Sustained Seawater Oxidation.ACS Appl Mater Interfaces. 2022 May 11;14(18):20443-20454. doi: 10.1021/acsami.1c24304. Epub 2022 Feb 9. ACS Appl Mater Interfaces. 2022. PMID: 35138809 Review.
-
Corrosion-resistant single-atom catalysts for direct seawater electrolysis.Natl Sci Rev. 2025 Feb 22;12(4):nwaf060. doi: 10.1093/nsr/nwaf060. eCollection 2025 Apr. Natl Sci Rev. 2025. PMID: 40171000 Free PMC article. Review.
-
Impact of harmful ions in seawater on electrolysis catalysts: challenges and mitigation strategies.Chem Commun (Camb). 2025 Apr 11;61(31):5719-5730. doi: 10.1039/d5cc00844a. Chem Commun (Camb). 2025. PMID: 40130362 Review.
-
The critical effect of different additive interlayer anions on NiFe-LDH for direct seawater splitting: A theoretical study.J Colloid Interface Sci. 2025 Feb 15;680(Pt B):43-52. doi: 10.1016/j.jcis.2024.11.069. Epub 2024 Nov 15. J Colloid Interface Sci. 2025. PMID: 39550852
-
High-entropy NiFeCoV disulfides for enhanced alkaline water/seawater electrolysis.J Colloid Interface Sci. 2023 Sep;645:724-734. doi: 10.1016/j.jcis.2023.04.172. Epub 2023 May 6. J Colloid Interface Sci. 2023. PMID: 37172482
Cited by
-
Anion Exchange Membrane Seawater Electrolysis at 1.0 A cm-2 With an Anode Catalyst Stable for 9000 H.Adv Sci (Weinh). 2025 Jun;12(22):e2416661. doi: 10.1002/advs.202416661. Epub 2025 Mar 28. Adv Sci (Weinh). 2025. PMID: 40153265 Free PMC article.
-
Anion-Exchange-Membrane Electrolysis with Alkali-Free Water Feed.Chem Rev. 2025 Aug 13;125(15):6906-6976. doi: 10.1021/acs.chemrev.4c00466. Epub 2025 Aug 1. Chem Rev. 2025. PMID: 40748968 Free PMC article. Review.
References
-
- Yu Z. Y., Duan Y., Feng X. Y., Yu X., Gao M. R., Yu S. H., Adv. Mater. 2021, 33, 2007100. - PubMed
-
- Ran J., Jaroniec M., Qiao S. Z., Adv. Mater. 2018, 30, 1704649. - PubMed
-
- Abdul Nasir J., Munir A., Ahmad N., ul Haq T., Khan Z., Rehman Z., Adv. Mater. 2021, 2105195. - PubMed
-
- Haq T. U., Haik Y., Hussain I., Rehman H. U., Al-Ansari T. A., ACS Appl. Mater. Interfaces 2021, 13, 468. - PubMed
LinkOut - more resources
Full Text Sources
