Obesity, overweight and breast cancer: new clinical data and implications for practice
- PMID: 40212682
- PMCID: PMC11983443
- DOI: 10.3389/fonc.2025.1579876
Obesity, overweight and breast cancer: new clinical data and implications for practice
Abstract
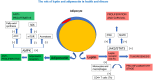
Excess bodyweight has negative consequences in breast cancer (BC) patients, significantly increasing the incidence of BC and adversely affecting clinical outcomes in most BC subtypes. This article overviews recent evidence relating to excess bodyweight (particularly obesity) and its effect on treatment in women with BC, focusing on latest evidence, including clinical findings from recently introduced new therapeutic entities. There is evidence of an inverse relationship between obesity and BC in premenopausal women highlighting a complex interplay involving the tumor microenvironment and tumor cells, and patient factors such as hormonal/metabolic/inflammatory status. Advancements in targeted- and immune-therapy have brought renewed optimism for women with BC. Ultimately, a better understanding of the mechanistic link between adipogenicity and tumorigenicity in breast tissues, as well as how obesity and adipose tissue inflammation interact with female sex hormones, may prove to be an important area for further refinements in our quest to develop a truly personalized therapeutic approach in this clinical setting.
Keywords: bodyweight; breast cancer; leptin; microenvironment; obesity.
Copyright © 2025 García-Estévez, González-Rodríguez, Calvo, Orta, Gión, Moreno-Bueno, Pérez-García and Cortés.
Conflict of interest statement
JP-G reports a consulting or advisory role with Lilly, Roche, Eisai, Daichii Sankyo, AstraZeneca, Seattle Genetics; and employment at MEDSIR. JC reports consulting/advisor from: Roche, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Lilly, MerckSharp&Dohme, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, Scorpion Therapeutics, Expres2ion Biotechnologies, Jazz Pharmaceuticals, Abbvie, Bridgebio, Biontech; honoraria from: Roche, Novartis, Eisai, Pfizer, Lilly, MerckSharp&Dohme, Daiichi Sankyo, AstraZeneca, Gilead, Steamline Therapeutics; research funding to the Institution: Roche, Ariad pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F. Hoffman-La Roche, Guardant health, MerckSharp&Dohme, Pfizer, Piqur Therapeutics, Iqvia, Queen Mary University of London. Stock: MAJ3 Capital, Leuko relative; travel, accommodation, expenses from: Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, Astrazeneca, Gilead, MerckSharp&Dohme, Steamline Therapeutics. Patents: Pharmaceutical Combinations of A Pi3k Inhibitor And A Microtubule Destabilizing Agent. JC Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A. ISSUED Her2 as a predictor of response to dualHER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/0,338,368 A1. LICENSED. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Obesity (2024). Available online at: https://www.who.int/health-topics/obesitytab=tab_1 (Accessed January 1, 2024).
-
- Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. (2016) 22:s176–85. - PubMed
-
- World Obesity Federation . World Obesity Atlas (2023). Available online at: https://www.worldobesity.org/resources/resource-library/world-obesity-at... (Accessed January 5, 2024).
Publication types
LinkOut - more resources
Full Text Sources

