Emerging Multifunctional Carbon-Nanomaterial-Based Biosensors for Cancer Diagnosis
- PMID: 40212699
- PMCID: PMC11935140
- DOI: 10.1002/smsc.202300221
Emerging Multifunctional Carbon-Nanomaterial-Based Biosensors for Cancer Diagnosis
Abstract
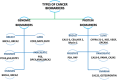
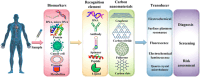
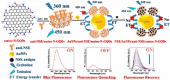
Despite significant advancements in medical technology, cancer remains the world's second-leading cause of death, largely attributed to late-stage diagnoses. While traditional cancer detection methodologies offer foundational insights, they often lack the specificity, affordability, and sensitivity for early-stage identification. In this context, the development of biosensors offers a distinct possibility for the precise and rapid identification of cancer biomarkers. Carbon nanomaterials, including graphene, carbon nitride, carbon quantum dots, and other carbon-based nanostructures, are highly promising for cancer detection. Their simplicity, high sensitivity, and cost-effectiveness contribute to their potential in this field. This review aims to elucidate the potential of emerging carbon-nanomaterial-based biosensors for early cancer diagnosis. The relevance of the various biosensor mechanisms and their performance to the physicochemical properties of carbon nanomaterials is discussed in depth, focusing on demonstrating broad methodologies for creating performance biosensors. Diverse carbon-nanomaterial-based detection techniques, such as electrochemical, fluorescence, surface plasmon resonance, electrochemiluminescence, and quartz crystal microbalance, are emphasized for early cancer detection. At last, a summary of existing challenges and future outlook in this promising field is elaborated.
Keywords: biomarkers; biosensors; cancer diagnosis; carbon nanomaterials; detection.
© 2023 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Recent Progress in Graphene- and Related Carbon-Nanomaterial-based Electrochemical Biosensors for Early Disease Detection.ACS Biomater Sci Eng. 2022 Mar 14;8(3):964-1000. doi: 10.1021/acsbiomaterials.1c00710. Epub 2022 Mar 1. ACS Biomater Sci Eng. 2022. PMID: 35229605 Review.
-
Recent advances in nanomaterial-based biosensors for the detection of exosomes.Anal Bioanal Chem. 2021 Jan;413(1):83-102. doi: 10.1007/s00216-020-03000-0. Epub 2020 Nov 8. Anal Bioanal Chem. 2021. PMID: 33164151 Review.
-
Carbon-based biosensors from graphene family to carbon dots: A viewpoint in cancer detection.Talanta. 2023 Jun 1;258:124399. doi: 10.1016/j.talanta.2023.124399. Epub 2023 Feb 28. Talanta. 2023. PMID: 36870153 Review.
-
Recent achievements in exosomal biomarkers detection by nanomaterials-based optical biosensors - A review.Anal Chim Acta. 2020 Jun 1;1114:74-84. doi: 10.1016/j.aca.2020.02.041. Epub 2020 Feb 24. Anal Chim Acta. 2020. PMID: 32359518 Review.
-
Nanomaterial-based biosensors for the detection of foodborne bacteria: a review.Analyst. 2023 Nov 20;148(23):5790-5804. doi: 10.1039/d3an01554h. Analyst. 2023. PMID: 37855707 Review.
Cited by
-
Nanomaterial-Based Biosensors for the Detection of COVID-19.Indian J Microbiol. 2025 Mar;65(1):120-136. doi: 10.1007/s12088-024-01336-0. Epub 2024 Jun 23. Indian J Microbiol. 2025. PMID: 40371045
References
LinkOut - more resources
Full Text Sources
