The double-edged sword of nutraceuticals: comprehensive review of protective agents and their hidden risks
- PMID: 40212723
- PMCID: PMC11984464
- DOI: 10.3389/fnut.2025.1524627
The double-edged sword of nutraceuticals: comprehensive review of protective agents and their hidden risks
Abstract
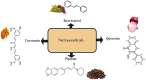
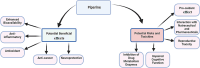
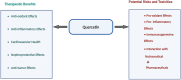
Nutraceuticals-including resveratrol (RSV), curcumin (CUR), piperine (PPR), and quercetin (QUE)-exhibit dual therapeutic and toxicological profiles, are necessitating balanced risk-benefit evaluation. This review synthesizes evidence from about 120 preclinical/clinical studies sourced from PubMed, Scopus, and Web of Science using keywords (e.g., nutraceutical-drug interactions, bioavailability, CYP/P-gp modulation), prioritizing recent advances (2015-2024) alongside seminal works to contextualize mechanisms. Studies were selected based on methodological rigor, clinical relevance, and mechanistic insights into protective effects (antioxidant, anti-inflammatory, anticancer) and risks (organ toxicity, pro-oxidant activity, drug interactions). Key findings highlight PPR's bioavailability-enhancing and neuroprotective properties, yet its inhibition of CYP3A4/P-gp elevates toxicity risks for carbamazepine (68.7% ↑ plasma concentration) and warfarin. CUR demonstrates hepatoprotective benefits but alters cardiovascular drug pharmacokinetics (e.g., amlodipine) and induces oxidative stress at high doses. RSV and QUE improve cardiovascular/neurological outcomes but interact with chemotherapeutics (RSV ↓ drug resistance via apoptosis; QUE ↑ methotrexate efficacy via anti-inflammatory synergy). Critical risks include reproductive toxicity (PPR >10 mg/kg), neurocognitive deficits (high-dose CUR), and CYP3A4-mediated interactions (QUE + cyclosporine). Nanotechnology-driven formulations (e.g., CUR/PPR nanoemulsions) mitigate risks by enhancing stability and enabling targeted delivery, though rigorous safety validation remains essential. This review underscores the need for evidence-based guidelines to optimize nutraceutical use in polypharmacy populations, emphasizing interdisciplinary collaboration to manage interactions. Innovations like nanoencapsulation could transition nutraceuticals from supplements to precision medicine adjuvants, pending resolution of dose-response ambiguities and long-term safety gaps through targeted research.
Keywords: curcumin; nutraceuticals; nutraceuticals-pharmaceutical interactions; piperine; quercetin; resveratrol.
Copyright © 2025 Ashrafpour and Ashrafpour.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Curcumin and quercetin synergistically attenuate subacute diazinon-induced inflammation and oxidative neurohepatic damage, and acetylcholinesterase inhibition in albino rats.Environ Sci Pollut Res Int. 2019 Feb;26(4):3659-3665. doi: 10.1007/s11356-018-3907-9. Epub 2018 Dec 8. Environ Sci Pollut Res Int. 2019. PMID: 30535736
-
A Critical Appraisal of the Protective Activity of Polyphenolic Antioxidants against Iatrogenic Effects of Anticancer Chemotherapeutics.Antioxidants (Basel). 2024 Jan 22;13(1):133. doi: 10.3390/antiox13010133. Antioxidants (Basel). 2024. PMID: 38275658 Free PMC article. Review.
-
The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial.Front Nutr. 2023 Jan 18;9:1023997. doi: 10.3389/fnut.2022.1023997. eCollection 2022. Front Nutr. 2023. PMID: 36742008 Free PMC article.
-
Anti- and pro-oxidant effects of oxidized quercetin, curcumin or curcumin-related compounds with thiols or ascorbate as measured by the induction period method.In Vivo. 2006 Jan-Feb;20(1):39-44. In Vivo. 2006. PMID: 16433026
Cited by
-
Antioxidants and Reactive Oxygen Species: Shaping Human Health and Disease Outcomes.Int J Mol Sci. 2025 Aug 4;26(15):7520. doi: 10.3390/ijms26157520. Int J Mol Sci. 2025. PMID: 40806653 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

