Synergistic effects of electrical and chemical cues with biodegradable scaffolds for large peripheral nerve defect regeneration
- PMID: 40212780
- PMCID: PMC11985093
- DOI: 10.1016/j.bioactmat.2025.03.017
Synergistic effects of electrical and chemical cues with biodegradable scaffolds for large peripheral nerve defect regeneration
Abstract
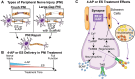
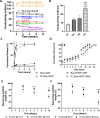
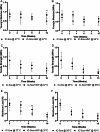
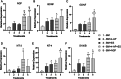
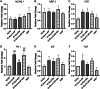
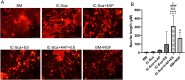
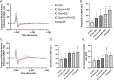
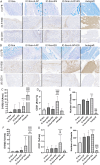
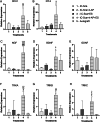
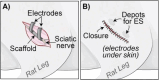
Large-gap peripheral nerve injuries (PNI) are often treated with autografts, allografts, or synthetic grafts to facilitate nerve regeneration, but these options are often limited in their availability or functionality. To address these issues, we developed ionically conductive (IC) nerve guidance conduits (NGCs) of sufficient biodegradability, mechanical strength, and bioactivity to support large-gap nerve regeneration. These chitosan-based NGCs release 4-aminopyridine (4-AP) from embedded halloysite nanotubes, and the NGC's IC properties enable transcutaneous electrical stimulation (ES) without invasive electrodes. In vitro, we found scaffolds with ES+4-AP synergistically enhanced Schwann cell adhesion, proliferation, and neurotrophin secretion, significantly improving axonal growth and neurite extension. In vivo, these scaffolds in large-gap PNI boosted neurotrophin levels, myelination, nerve function, and muscle weight while promoting angiogenesis and reducing fibrosis. Upregulated Trk receptors and PI3K/Akt and MAPK pathway highlight the regenerative potential. This study advances understanding of ES-mediated regeneration and supports innovative strategies for nerve and musculoskeletal repair.
Keywords: 4-Aminopyridine (4-AP); Ionically conductive nerve conduits; Neurotrophic factors; Peripheral nerve regeneration; Schwann cell proliferation; Sciatic nerve injury repair; Transcutaneous electrical stimulation.
© 2025 The Authors.
Conflict of interest statement
Corresponding author Sangamesh G. Kumbar is an Associate Editor for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures







References
-
- Brattain K. Magellan Medical Technology Consultants, Inc.; 2013. ANALYSIS OF THE PERIPHERAL NERVE REPAIR MARKET IN THE UNITED STATES.
-
- Chrząszcz P., Derbisz K., Suszyński K., Miodoński J., Trybulski R., Lewin-Kowalik J., Marcol W. Application of peripheral nerve conduits in clinical practice: a literature review. Neurol. Neurochir. Pol. 2018;52(4):427–435. - PubMed
-
- Manoukian O.S., Baker J.T., Rudraiah S., Arul M.R., Vella A.T., Domb A.J., Kumbar S.G. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J. Contr. Release. 2020;317:78–95. - PubMed
-
- Shapira Y., Sammons V., Forden J., Guo G.F., Kipp A., Girgulis J., Mishra T., de Villers Alant J.D., Midha R. Brief electrical stimulation promotes nerve regeneration following experimental in-continuity nerve injury. Neurosurgery. 2019;85(1):156–163. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

