Impact of the Coronavirus Disease 2019 Pandemic on Initiation Therapy for Noninfectious Uveitis
- PMID: 40212929
- PMCID: PMC11985044
- DOI: 10.1016/j.xops.2025.100718
Impact of the Coronavirus Disease 2019 Pandemic on Initiation Therapy for Noninfectious Uveitis
Abstract
Purpose: Initial studies during the coronavirus disease 2019 (COVID-19) pandemic demonstrated a possible increased risk of COVID-19 infection and severe outcomes with prior or concurrent immunomodulatory therapy (IMT). The purpose of this study was to determine the impact of the COVID-19 pandemic on treatment patterns for noninfectious uveitis (NIU).
Design: Retrospective interrupted time series (ITS) analysis using Optum Labs Data Warehouse, a national deidentified health care database in the United States with administrative claims and electronic health record data.
Participants: Individuals with a new diagnosis of NIU from December 1, 2017, to December 31, 2020, with continuous enrollment ≥1 year before this diagnosis.
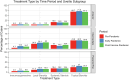
Methods: This study was divided into 3 time periods: prepandemic (December 1, 2017-November 30, 2019), early pandemic (March 1, 2020-December 31, 2020), and postvaccine period (January 1, 2021-September 30, 2021) corresponding to time before the pandemic, during the pandemic when no COVID-19 vaccine was available, and after widespread utilization of the vaccine began. Normalized prescription rates of uveitis therapies were modeled as an ITS. In the time-to-treatment analysis, Cox proportional hazard models were used to determine differences in likelihood of different modalities between time periods.
Main outcome measures: Temporal trends in the initial therapeutic choice for NIU.
Results: This study included 22 444 patients with a new NIU diagnosis. The average age was 61.9 (standard deviation 17.5) years, and 59.3% were female. There were no significant temporal breaks in prescribing trends for topical, local, and systemic corticosteroids or immunosuppressive therapy (disease-modifying antirheumatic drugs and biologics) between pandemic periods (all P > 0.05) in ITS analysis. Overall, topical steroids were more likely to be prescribed in the early versus prepandemic period (hazard ratio [HR] 1.10; 95% confidence interval [CI] 1.06-1.15; P < 0.001). Intraocular steroids also saw greater relative use during the early (HR 1.29; 95% CI 1.13-1.46; P < 0.001) and postvaccine (HR 1.29; 95% CI 1.14-1.46; P < 0.001) period. Use of IMTs increased in the postvaccine period compared with that in the prepandemic period (HR 1.25; 95% CI 1.07-1.46; P < 0.001).
Conclusions: No significant differences in prescribing patterns for NIU were observed between pandemic periods. However, utilization of topical and local steroids for NIU was, overall, increased in the early compared with the prepandemic period.
Financial disclosures: The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Keywords: COVID-19; Inflammation; Treatment medical; Uveitis.
© 2025 by the American Academy of Ophthalmologyé.
Figures
Similar articles
-
Immunosuppressive Medications and COVID-19 Outcomes in Patients with Noninfectious Uveitis in the Era of COVID-19 Vaccinations.Ophthalmol Sci. 2023 Oct 13;4(2):100411. doi: 10.1016/j.xops.2023.100411. eCollection 2024 Mar-Apr. Ophthalmol Sci. 2023. PMID: 38146526 Free PMC article.
-
The Association between Noninfectious Uveitis and Coronavirus Disease 2019 Outcomes: An Analysis of United States Claims-Based Data.Ophthalmology. 2022 Mar;129(3):334-343. doi: 10.1016/j.ophtha.2021.10.007. Epub 2021 Oct 11. Ophthalmology. 2022. PMID: 34648828 Free PMC article.
-
Risk of Noninfectious Uveitis after Coronavirus Disease 2019 Vaccination in a United States Claims Database.Ophthalmology. 2023 Dec;130(12):1269-1278. doi: 10.1016/j.ophtha.2023.07.017. Epub 2023 Jul 20. Ophthalmology. 2023. PMID: 37480943 Free PMC article.
-
New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review.Semin Arthritis Rheum. 2019 Dec;49(3):438-445. doi: 10.1016/j.semarthrit.2019.06.004. Epub 2019 Jun 10. Semin Arthritis Rheum. 2019. PMID: 31301816 Review.
-
Local versus Systemic Therapy for Noninfectious Uveitis (NIU).Semin Ophthalmol. 2023 Jan;38(1):15-23. doi: 10.1080/08820538.2022.2152707. Epub 2022 Dec 5. Semin Ophthalmol. 2023. PMID: 36471661 Review.
References
-
- Tan E.H., Sena A.G., Prats-Uribe A., et al. Characteristics, outcomes, and mortality amongst 133,589 patients with prevalent autoimmune diseases diagnosed with, and 48,418 hospitalised for COVID-19: a multinational distributed network cohort analysis. medRxiv. 2020;2020 doi: 10.1101/2020.11.24.20236802. - DOI
-
- Pablos J.L., Galindo M., Carmona L., et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79:1544–1549. - PubMed
LinkOut - more resources
Full Text Sources