Noncovalent Immobilization of Pentamethylcyclopentadienyl Iridium Complexes on Ordered Mesoporous Carbon for Electrocatalytic Water Oxidation
- PMID: 40212958
- PMCID: PMC11935816
- DOI: 10.1002/smsc.202100037
Noncovalent Immobilization of Pentamethylcyclopentadienyl Iridium Complexes on Ordered Mesoporous Carbon for Electrocatalytic Water Oxidation
Abstract
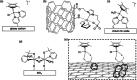
The attachment of molecular catalysts to conductive supports for the preparation of solid-state anodes is important for the development of devices for electrocatalytic water oxidation. The preparation and characterization of three molecular cyclopentadienyl iridium(III) complexes, Cp*Ir(1-pyrenyl(2-pyridyl)ethanolate-κO,κN)Cl (1) (Cp* = pentamethylcyclopentadienyl), Cp*Ir(diphenyl(2-pyridyl)methanolate-κO,κN)Cl (2), and [Cp*Ir(4-(1-pyrenyl)-2,2'-bipyridine)Cl]Cl (3), as precursors for electrochemical water oxidation catalysts, are reported. These complexes contain aromatic groups that can be attached via noncovalent π-stacking to ordered mesoporous carbon (OMC). The resulting iridium-based OMC materials (Ir-1, Ir-2, and Ir-3) were tested for electrocatalytic water oxidation leading to turnover frequencies (TOFs) of 0.9-1.6 s-1 at an overpotential of 300 mV under acidic conditions. The stability of the materials is demonstrated by electrochemical cycling and X-ray absorption spectroscopy analysis before and after catalysis. Theoretical studies on the interactions between the molecular complexes and the OMC support provide insight onto the noncovalent binding and are in agreement with the experimental loadings.
Keywords: electrocatalysis; iridium molecular complexes; noncovalent immobilization; ordered mesoporous carbon; water oxidation.
© 2021 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Characterization of an amorphous iridium water-oxidation catalyst electrodeposited from organometallic precursors.Inorg Chem. 2013 Feb 18;52(4):1860-71. doi: 10.1021/ic301968j. Epub 2013 Feb 5. Inorg Chem. 2013. PMID: 23383971
-
Heterogeneous Catalysis for Water Oxidation by an Iridium Complex Immobilized on Bipyridine-Periodic Mesoporous Organosilica.Angew Chem Int Ed Engl. 2016 Jul 4;55(28):7943-7. doi: 10.1002/anie.201601453. Epub 2016 May 11. Angew Chem Int Ed Engl. 2016. PMID: 27168492
-
Electrocatalytic carbon dioxide reduction by using cationic pentamethylcyclopentadienyl-iridium complexes with unsymmetrically substituted bipyridine ligands.Chemistry. 2015 Apr 20;21(17):6564-71. doi: 10.1002/chem.201404367. Epub 2015 Mar 10. Chemistry. 2015. PMID: 25756194
-
Recent Trends on Electrochemical Sensors Based on Ordered Mesoporous Carbon.Sensors (Basel). 2017 Aug 11;17(8):1863. doi: 10.3390/s17081863. Sensors (Basel). 2017. PMID: 28800106 Free PMC article. Review.
-
The Enhanced Catalytic Performance and Stability of Ordered Mesoporous Carbon Supported Nano-Gold with High Structural Integrity for Glycerol Oxidation.Chem Rec. 2019 Sep;19(9):1913-1925. doi: 10.1002/tcr.201800109. Epub 2018 Nov 21. Chem Rec. 2019. PMID: 30462369 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
