Interfaces in Atomic Layer Deposited Films: Opportunities and Challenges
- PMID: 40213138
- PMCID: PMC11935996
- DOI: 10.1002/smsc.202300060
Interfaces in Atomic Layer Deposited Films: Opportunities and Challenges
Abstract
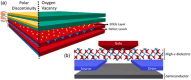
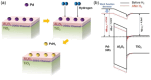
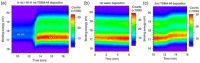
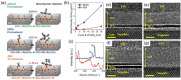
Atomic layer deposition (ALD) is an effective method for precise layer-wise growth of thin-film materials and has allowed for substantial progress in a variety of fields. Advances in the technique have instigated high-level interpretations of the relationship between nanostructure architecture and performance. An inherent part in the ALD of films is the underlying interfaces between each material, which plays a significant role in advanced electronics. Considering the impact of sandwiched substructures, it is appropriate to highlight opportunities and challenges faced by applications that rely on these interfaces. This review encompasses the current prospects and obstacles to further performance improvements in ALD-generated interfaces. 2D electron gas, high-k materials, freestanding layered structures, lattice matching, and seed layers, as well as prospects for future research, are explored.
Keywords: 2D electron gas; atomic layer deposition; freestanding 2D layers; high-k layers; seeding layers.
© 2023 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

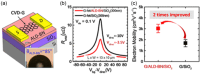
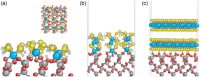


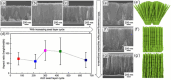

References
-
- Seshan K., Schepis D., Handbook of Thin Film Deposition, William Andrew, Norwich, NY, USA: 2018.
-
- Baisnab D. K., Mukherjee S., Das S., in Chemical Solution Synthesis for Materials Design and Thin Film Device Applications (Eds: Das S., Dhara S.), Elsevier, Amsterdam, The Netherlands: 2021, pp. 231–275, 10.1016/B978-0-12-819718-9.00007-8. - DOI
-
- Putkonen M., Atomic Layer Deposition of Nanostructured Materials, John Wiley & Sons, Ltd., Hoboken, NJ, USA: 2011, pp. 41–59.
-
- Zaidi S. J. A., Basit M. A., Park T. J., Chem. Mater. 2022, 34, 7106.
-
- Short A., Jewell L., Bielecki A., Keiber T., Bridges F., Carter S., Alers G., J. Vac. Sci. Technol., A 2014, 32, 01A125.
LinkOut - more resources
Full Text Sources
