Vertex-Shared Linear Superatomic Molecules: Stepping Stones to Novel Materials Composed of Noble Metal Clusters
- PMID: 40213321
- PMCID: PMC11935948
- DOI: 10.1002/smsc.202300024
Vertex-Shared Linear Superatomic Molecules: Stepping Stones to Novel Materials Composed of Noble Metal Clusters
Abstract
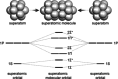
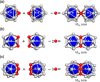
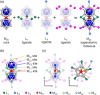
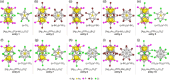
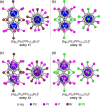
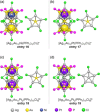
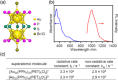
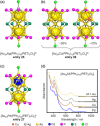
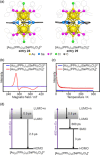
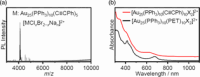
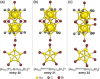
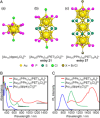
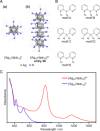
Extremely small metal clusters composed of noble metal atoms (M) have orbitals similar to those of atoms and therefore can be thought of as artificial atoms or superatoms. If these superatoms can be assembled into molecular analogs, it might be possible to create materials with new characteristics and properties that are different from those of existing substances. Therefore, the concept of superatomic molecules has attracted significant attention. The present review focuses on vertex-shared linear M12n+1 superatomic molecules formed via the sharing of a single metal atom between M13 superatoms having icosahedral cores and summarizes the knowledge obtained to date in this regard. This summary discusses the most suitable ligand combinations for the synthesis of M12n+1 superatomic molecules along with the valence electron numbers, stability, optical absorption characteristics, and luminescence properties of the M12n+1 superatomic molecules fabricated to date. This information is expected to assist in the production of many M12n+1 superatomic molecules with novel structures and physicochemical properties in the future.
Keywords: connections; geometrical structures; ligand-protected metal clusters; superatomic molecules.
© 2023 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Key factors for connecting silver-based icosahedral superatoms by vertex sharing.Commun Chem. 2023 Mar 28;6(1):57. doi: 10.1038/s42004-023-00854-0. Commun Chem. 2023. PMID: 36977829 Free PMC article.
-
Superatomic Aromaticity in Cyclic Superatomic Molecules: Ligand-Protected Penta-Icosahedral [M@Au11]5 (M = Au, Pt) Clusters.J Phys Chem A. 2024 Apr 18;128(15):2982-2988. doi: 10.1021/acs.jpca.4c00229. Epub 2024 Apr 5. J Phys Chem A. 2024. PMID: 38578691
-
Special and general superatoms.Acc Chem Res. 2014 Oct 21;47(10):2931-40. doi: 10.1021/ar5001583. Epub 2014 Sep 24. Acc Chem Res. 2014. PMID: 25252219
-
Insight into the Geometric and Electronic Structures of Gold/Silver Superatomic Clusters Based on Icosahedron M13 Units and Their Alloys.Chem Asian J. 2019 Oct 1;14(19):3222-3231. doi: 10.1002/asia.201900760. Epub 2019 Sep 3. Chem Asian J. 2019. PMID: 31368672 Review.
-
Systematics of stable copper and silver clusters protected by small bite chelating bidentate sulfur and selenium ligands related to their polyhedral cavities: analogies to aliphatic compounds and three-dimensional spherical aromatic systems.Dalton Trans. 2024 Feb 13;53(7):2895-2902. doi: 10.1039/d3dt03998f. Dalton Trans. 2024. PMID: 38170867 Review.
References
-
- Cohen M. L., Knight W. D., Phys. Today 1990, 43, 42.
-
- de Heer W. A., Rev. Mod. Phys. 1993, 65, 611.
-
- Briant C. E., Theobald B. R. C., White J. W., Bell L. K., Mingos D. M. P., Welch A. J., J. Chem. Soc., Chem. Commun. 1981, 201.
-
- a) Shichibu Y., Konishi K., Small 2010, 6, 1216; - PubMed
- b) Zhang J., Zhou Y., Zheng K., Abroshan H., Kauffman D. R., Sun J., Li G., Nano Res. 2018, 11, 5787.
LinkOut - more resources
Full Text Sources
