Coordination-driven assembly of a ferrocene-functionalized lead iodide framework with enhanced stability and charge transfer for photocatalytic CO2-to-CH3OH conversion
- PMID: 40213370
- PMCID: PMC11979706
- DOI: 10.1039/d4sc08216h
Coordination-driven assembly of a ferrocene-functionalized lead iodide framework with enhanced stability and charge transfer for photocatalytic CO2-to-CH3OH conversion
Abstract
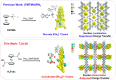
Hybrid lead halides are promising photocatalysts due to their high structural tunability and excellent photophysical properties, but their ionic structures suffer from instability in polar environments and suppressed charge transfer between lead halide units and organic components. Herein, we successfully incorporated a ferrocene-based light-harvesting antenna into a lead iodide framework by coordination-driven assembly. The π-conjugated Pb2+-carboxylate linkage affords synergistic interactions between [Pb2I2]2+ chains and ferrocene linkers, achieving broad visible absorption up to 612.7 nm and efficient ligand-to-metal charge transfer for spatial charge separation. This ultrastable framework combines strong visible-light absorption of ferrocene centers with excellent charge transport of lead halide units, achieving 6e- CO2 photoreduction to CH3OH coupled with ethanol oxidation. Mechanistic studies reveal that ferrocene photoexcitation followed by linker-to-metal charge transfer significantly enhances carrier accumulation, accelerating CH3O* intermediate formation as indicated by in situ spectroscopy and theoretical calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Organolead Halide-Based Coordination Polymers: Intrinsic Stability and Photophysical Applications.Acc Chem Res. 2023 Feb 21;56(4):452-461. doi: 10.1021/acs.accounts.2c00687. Epub 2023 Jan 31. Acc Chem Res. 2023. PMID: 36719833
-
A layered lead halide framework intercalated with Ru(bpy)3 for efficient CO2 photoreduction.Nat Commun. 2025 Jul 1;16(1):5910. doi: 10.1038/s41467-025-60954-4. Nat Commun. 2025. PMID: 40593614 Free PMC article.
-
Modulating Inorganic Dimensionality of Ultrastable Lead Halide Coordination Polymers for Photocatalytic CO2 Reduction to Ethanol.Angew Chem Int Ed Engl. 2024 Apr 15;63(16):e202316080. doi: 10.1002/anie.202316080. Epub 2024 Mar 7. Angew Chem Int Ed Engl. 2024. PMID: 38385586
-
Metal-Organic-Framework-Based Catalysts for Photoreduction of CO2.Adv Mater. 2018 Aug;30(35):e1705512. doi: 10.1002/adma.201705512. Epub 2018 Jun 12. Adv Mater. 2018. PMID: 29894012 Review.
-
Metal-Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production.Angew Chem Int Ed Engl. 2016 Apr 25;55(18):5414-45. doi: 10.1002/anie.201505581. Epub 2016 Mar 11. Angew Chem Int Ed Engl. 2016. PMID: 26970539 Review.
References
-
- Graciani J. Mudiyanselage K. Xu F. Baber A. E. Evans J. Senanayake S. D. Stacchiola D. J. Liu P. Hrbek J. Sanz J. F. Rodriguez J. A. Science. 2014;345:546–550. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

