Floating Interlayer and Surface Electrons in 2D Materials: Graphite, Electrides, and Electrenes
- PMID: 40213406
- PMCID: PMC11935841
- DOI: 10.1002/smsc.202100020
Floating Interlayer and Surface Electrons in 2D Materials: Graphite, Electrides, and Electrenes
Abstract
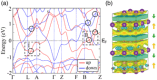
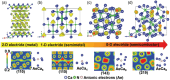
Over the last half century, layered materials have been at the forefront of materials science, spearheading the discovery of new phenomena and functionalities. Certain layered materials are known to possess electronic states unassociated with any of the constituent atoms, having a large proportion of their probability amplitude in the space between the layers. Usually, such a nucleus-free interlayer state has energy above the Fermi level and is unoccupied. However, the energy decreases when cations are intercalated and may cross the Fermi level, as in the case of C6Ca, a superconductor with a T c of 11.5 K. A major thrust to the research of interlayer electrons comes with the discovery of layered electrides, which are alternating stacks of positively charged ionic layers and negatively charged sheets of electrons in the interlayer space. When intercalation compounds and layered electrides are thinned down to the atomic scale, the interlayer states survive as surface states floating over the surface. This review provides a unified overview of the two classes of materials hosting interlayer floating electrons near the Fermi level, intercalation compounds and layered electrides, and their properties, including high electron mobility, low work function, ultralow interlayer friction, superconductivity, and plasmonic properties.
Keywords: 2D materials; electrides; graphite; layered materials; surface state; work functions.
© 2021 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















References
-
- Posternak M., Baldereschi A., Freeman A. J., Wimmer E., Weinert M., Phys. Rev. Lett. 1983, 50, 761.
-
- Ohno T., Nakao K., Kamimura H., J. Phys. Soc. Jpn. 1979, 47, 1125.
-
- Kamimura H., Nakao K., Ohno T., Inoshita T., Phys. B+C 1980, 99, 401.
-
- Catellani A., Posternak M., Baldereschi A., Jansen H. J. F., Freeman A. J., Phys. Rev. B 1985, 32, 6997. - PubMed
-
- Koma A., Miki K., Suematsu H., Ohno T., Kamimura H., Phys. Rev. B 1986, 34, 2434. - PubMed
LinkOut - more resources
Full Text Sources
