Improved CAR internalization and recycling through transmembrane domain optimization reduces CAR-T cytokine release and exhaustion
- PMID: 40213546
- PMCID: PMC11983635
- DOI: 10.3389/fimmu.2025.1531344
Improved CAR internalization and recycling through transmembrane domain optimization reduces CAR-T cytokine release and exhaustion
Abstract
Background: Anti-CD19 chimeric antigen receptor T (CAR-T) cell therapy has proven effective for treating relapsed or refractory acute B cell leukemia. However, challenges such as cytokine release syndrome, T cell dysfunction, and exhaustion persist. Enhancing CAR-T cell efficacy through changing CAR internalization and recycling is a promising approach. The transmembrane domain is the easiest motif to optimize for modulating CAR internalization and recycling without introducing additional domains, and its impact on CAR internalization and recycling has not yet been thoroughly explored. In this study, we aim to enhance CAR-T cell function by focusing on the solely transmembrane domain design.
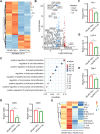
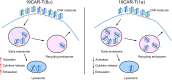
Methods: Utilizing plasmid construction and lentivirus generation, we get two different transmembrane CAR-T cells [19CAR-T(1a) and 19CAR-T(8α)]. Through co-culture with tumor cells, we evaluate CAR dynamic change, activation levels, exhaustion markers, mitochondrial function, and differentiation in both CAR-T cells. Furthermore, immunofluorescence microscopy analysis is performed to reveal the localization of internalized CAR molecules. RNA sequencing is used to detect the transcriptome of activated CAR-T cells. Finally, a mouse study is utilized to verify the anti-tumor efficacy of 19CAR-T(1a) cells in vivo.
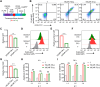
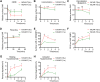
Results: Our findings demonstrate that 19CAR-T(1a) has lower surface CAR expression, faster internalization, and a higher recycling rate compared to 19CAR-T(8α). Internalized 19CAR(1a) co-localizes more with early and recycling endosomes, and less with lysosomes than 19CAR(8α). These features result in lower activation levels, less cytokine release, and reduced exhaustion markers in 19CAR-T(1a). Furthermore, CAR-T cells with CD1a transmembrane domain also exhibit a superior anti-tumor ability and reduced exhaustion in vivo.
Conclusion: Overall, we demonstrate that the transmembrane domain plays a critical role in CAR-T cell function. An optimized transmembrane domain can alleviate cytokine release syndrome and reduce CAR-T cell exhaustion, providing a direction for CAR design to enhance CAR-T cell function.
Keywords: car-t; cytokine release; exhaustion; internalization; recycling; transmembrane domain.
Copyright © 2025 Xie, Long, Wang, Xiang, Xian, Wang, Dou, Zhang, Li, Kang, Chen, Zhao, Xu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Reducing Hinge Flexibility of CAR-T Cells Prolongs Survival In Vivo With Low Cytokines Release.Front Immunol. 2021 Oct 5;12:724211. doi: 10.3389/fimmu.2021.724211. eCollection 2021. Front Immunol. 2021. PMID: 34675920 Free PMC article.
-
The impact of CD3ζ ITAM multiplicity and sequence on CAR T-cell survival and function.Front Immunol. 2025 Jan 16;15:1509980. doi: 10.3389/fimmu.2024.1509980. eCollection 2024. Front Immunol. 2025. PMID: 39885989 Free PMC article.
-
In vitro functional validation of anti-CD19 chimeric antigen receptor T cells expressing lysine-specific demethylase 1 short hairpin RNA for the treatment of diffuse large B cell lymphoma.Front Immunol. 2025 Jan 13;15:1521778. doi: 10.3389/fimmu.2024.1521778. eCollection 2024. Front Immunol. 2025. PMID: 39872520 Free PMC article.
-
Recent advances in CAR-T cell engineering.J Hematol Oncol. 2020 Jul 2;13(1):86. doi: 10.1186/s13045-020-00910-5. J Hematol Oncol. 2020. PMID: 32616000 Free PMC article. Review.
-
Chimeric Antigen Receptor T-Cell Therapies for Aggressive B-Cell Lymphomas: Current and Future State of the Art.Am Soc Clin Oncol Educ Book. 2019 Jan;39:446-453. doi: 10.1200/EDBK_238693. Epub 2019 May 17. Am Soc Clin Oncol Educ Book. 2019. PMID: 31099671 Review.
Cited by
-
Introducing CAR-T Therapy in Kazakhstan: Establishing Academic-Scale Lentiviral Vector and CAR-T Cell Production.Biomolecules. 2025 Aug 14;15(8):1166. doi: 10.3390/biom15081166. Biomolecules. 2025. PMID: 40867610 Free PMC article.
-
Utilization of universal-targeting mSA2 CAR-T cells for the treatment of glioblastoma.Oncoimmunology. 2025 Dec;14(1):2518631. doi: 10.1080/2162402X.2025.2518631. Epub 2025 Jun 15. Oncoimmunology. 2025. PMID: 40518621 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

