Boosting the Solar Water Oxidation Performance of Fe2O3 Photoanode via Embedding Laser-Generated Pt Nanocrystals
- PMID: 40213568
- PMCID: PMC11935253
- DOI: 10.1002/smsc.202300318
Boosting the Solar Water Oxidation Performance of Fe2O3 Photoanode via Embedding Laser-Generated Pt Nanocrystals
Abstract
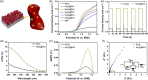
α-Fe2O3 with suitable band structure, good chemical stability, and easy preparation, is a potential photoanode material. However, the key to enhance the performance of α-Fe2O3 photoanode is to improve the transport characteristics of bulk carriers. It is expected to form a Schottky barrier to improve the carrier separation efficiency by embedding metal nanoparticles into the matrix, but the process is still challenging. Herein, a strategy of forming the Schottky barrier is shown to improve bulk carrier transport dynamics by embedding laser-generated Pt nanocrystals in α-Fe2O3 photoanode, which achieves photocurrent densities of up to 1.16 mA cm-2 at 1.23 VRHE (from original 0.21 mA cm-2). This work provides another way to promote the carrier transfer and separation of α-Fe2O3, which is of great significance to improve the photoelectrochemical water splitting performance.
Keywords: composite Fe2O3@Pt; nanocrystals embedding; nanoporous α‐Fe2O3; photoanodes; photoelectrochemical water splitting.
© 2024 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Embedding laser generated nanocrystals in BiVO4 photoanode for efficient photoelectrochemical water splitting.Nat Commun. 2019 Jun 13;10(1):2609. doi: 10.1038/s41467-019-10543-z. Nat Commun. 2019. PMID: 31197140 Free PMC article.
-
In Situ Synthesis of α-Fe2O3/Fe3O4 Heterojunction Photoanode via Fast Flame Annealing for Enhanced Charge Separation and Water Oxidation.ACS Appl Mater Interfaces. 2021 Jan 27;13(3):4785-4795. doi: 10.1021/acsami.0c19927. Epub 2021 Jan 12. ACS Appl Mater Interfaces. 2021. PMID: 33430580
-
Combining Bulk/Surface Engineering of Hematite To Synergistically Improve Its Photoelectrochemical Water Splitting Performance.ACS Appl Mater Interfaces. 2016 Jun 29;8(25):16071-7. doi: 10.1021/acsami.6b04142. Epub 2016 Jun 20. ACS Appl Mater Interfaces. 2016. PMID: 27275649
-
Enhanced photoelectrochemical water oxidation performance of a hematite photoanode by decorating with Au-Pt core-shell nanoparticles.Dalton Trans. 2017 Nov 28;46(46):16050-16057. doi: 10.1039/c7dt03838k. Dalton Trans. 2017. PMID: 29119164
-
Constructing Metal-Organic Framework Films with Adjustable Electronic Properties on Hematite Photoanode for Boosting Photogenerated Carrier Transport.Small. 2024 Nov;20(46):e2404438. doi: 10.1002/smll.202404438. Epub 2024 Aug 5. Small. 2024. PMID: 39101630
References
-
- Wang D., Fu Q., Tian J., Zhou H., Liu R., Zhan D., Peng Z., Han C., J. Colloid Interface Sci. 2024, 653, 1166. - PubMed
-
- Niu F., Wang D., Li F., Liu Y., Shen S., Meyer T. J., Adv. Energy Mater. 2019, 10, 1900399.
-
- Choi H., Seo S., Kim J.‐H., Lee J.‐H., Kim S., Piao G., Park H., Lee K., Lee S., J. Mater. Chem. A 2021, 9, 22291.
-
- Li S., Xu W., Meng L., Tian W., Li L., Small Sci. 2022, 2, 2100112.
LinkOut - more resources
Full Text Sources
