Functional Nucleic-Acid-Decorated Spherical Nanoparticles: Preparation Strategies and Current Applications in Cancer Therapy
- PMID: 40213613
- PMCID: PMC11935882
- DOI: 10.1002/smsc.202000056
Functional Nucleic-Acid-Decorated Spherical Nanoparticles: Preparation Strategies and Current Applications in Cancer Therapy
Abstract
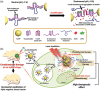
Functional nucleic acids (FNAs) have drawn widespread attention in the construction of functional nanomaterials for biomedical applications due to their inherent biological functions and sequence programmability, as well as high thermal stability and easy chemical modification. FNA-decorated spherical nanoparticles (FSNPs) are composed of a metal/metal-free spherical core and a radially oriented FNA shell. Attracted by their unique capabilities, such as resistance to nuclease degradation and capability of crossing the blood-brain barrier, FSNPs as smart nanomaterials for cancer therapy are reviewed. The preparation strategies of FSNPs are first summarized, and the applications of responsive linkers in stimuli-responsive drug release are introduced. The FSNPs are categorized into aptamer-, i-motif-, DNAzyme-, antisense oligonucleotide-, and CpG oligodeoxynucleotide-decorated SNPs. Their applications in cancer therapy include tumor-targeting drug delivery and controllable releasing of drugs, overcoming physiological or pathological obstacles such as blood-brain barrier and interstitial transport barriers, as well as a reversal of resistance to chemotherapy and antitumor immune response activation. The remaining challenges and future directions of FSNPs are also discussed and proposed.
Keywords: cancer therapy; controlled drug release; functional nucleic acids; nucleic acids linking strategies; spherical nanoparticles; targeted drug delivery.
© 2021 The Authors. Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications.Chem Soc Rev. 2016 Mar 7;45(5):1410-31. doi: 10.1039/c5cs00586h. Chem Soc Rev. 2016. PMID: 26758955 Free PMC article. Review.
-
Molecular Engineering of Functional Nucleic Acid Nanomaterials toward In Vivo Applications.Adv Healthc Mater. 2019 Mar;8(6):e1801158. doi: 10.1002/adhm.201801158. Epub 2019 Feb 6. Adv Healthc Mater. 2019. PMID: 30725526 Free PMC article. Review.
-
Framework Nucleic Acids: A Promising Vehicle for Small Molecular Cargos.Curr Drug Metab. 2023;24(5):353-366. doi: 10.2174/1389200224666230120124402. Curr Drug Metab. 2023. PMID: 36683364 Review.
-
Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks.Chem Sci. 2017 Aug 1;8(8):5769-5780. doi: 10.1039/c7sc01765k. Epub 2017 Jun 6. Chem Sci. 2017. PMID: 28989617 Free PMC article.
-
Light responsive nucleic acid for biomedical application.Exploration (Beijing). 2022 Apr 6;2(5):20210099. doi: 10.1002/EXP.20210099. eCollection 2022 Oct. Exploration (Beijing). 2022. PMID: 37325506 Free PMC article. Review.
Cited by
-
The nucleic acid reactions on the nanomaterials surface for biomedicine.J Nanobiotechnology. 2025 Apr 23;23(1):308. doi: 10.1186/s12951-025-03374-2. J Nanobiotechnology. 2025. PMID: 40269855 Free PMC article. Review.
References
-
- Sharifi M., Attar F., Saboury A. A., Akhtari K., Hooshmand N., Hasan A., El-Sayed M. A., Falahati M., J. Controlled Release 2019, 311–312, 170. - PubMed
-
- Sara H., Seyed Mohammad Gheibi H., Ahmad G., Mehrdad K., Kimya P., Majid D., Curr. Med. Chem. 2020, 27, 1.
-
- Alyassin Y., Sayed E. G., Mehta P., Ruparelia K., Arshad M. S., Rasekh M., Shepherd J., Kucuk I., Wilson P. B., Singh N., Chang M. W., Fatouros D. G., Ahmad Z., Drug Discovery Today 2020, 25, 1513. - PubMed
-
- El-Hammadi M. M., Arias J. L., Expert Opin. Ther. Pat. 2019, 29, 891. - PubMed
LinkOut - more resources
Full Text Sources
