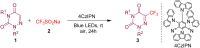The organophotocatalytic trifluoromethylation of 6-azauracils
- PMID: 40213620
- PMCID: PMC11984196
- DOI: 10.1039/d5ra00743g
The organophotocatalytic trifluoromethylation of 6-azauracils
Abstract
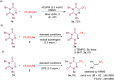
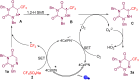
An efficient, cost-effective, and metal-free photocatalytic trifluoromethylation of 6-azauracils has been developed using Langlois reagent (CF3SO2Na) under ambient air. The present protocol, which utilizes an inexpensive CF3 reagent and an organophotocatalyst, provides a convenient way to prepare trifluoromethylated azauracil derivatives with a variety of functional groups. The experimental results suggest a radical mechanistic pathway for this methodology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
References
-
- Liu J. Gong Y. Shi J. Hao X. Wang Y. Zhou Y. Hou Y. Liu Y. Ding S. Chen Y. Eur. J. Med. Chem. 2020;194:112244. - PubMed
- Vanparijs O. Marsboom R. Van Der Flaes L. Poult. Sci. 1989;68:489. - PubMed
- Kishi T. Meltzer H. Y. Iwata N. Int. J. Neuropsychopharmacol. 2013;16:1259. - PubMed
- Deng N. Wu Y.-Y. Feng Y. Hsieh W.-C. Song J.-S. Lin Y.-S. Tseng Y.-H. Liao W.-J. Chu Y.-F. Liu Y.-C. Chang E.-C. Liu C.-R. Sheu S.-Y. Su M.-T. Kuo H.-C. Cohen S. N. Cheng T.-H. Proc. Natl. Acad. Sci. U. S. A. 2022;119:e2204779119. - PMC - PubMed
- Shaw T. A. Ablenas C. J. Desrochers G. F. Powdrill M. H. Bilodeau D. A. Vincent-Rocan J.-F. Niu M. Monette A. Mouland A. J. Beauchemin A. M. Pezacki J. P. Bioconjugate Chem. 2020;31:1537. - PubMed
-
- Crance J. M. Scaramozzino N. Jouan A. Garin D. Antiviral Res. 2003;58:73. - PubMed
- Duplantier A. J. Dombroski M. A. Subramanyam C. Beaulieu A. M. Chang S.-P. Gabel C. A. Jordan C. Kalgutkar A. S. Kraus K. G. Labasi J. M. Mussari C. Perregaux D. G. Shepard R. Taylor T. J. Trevena K. A. Whitney-Pickett C. Yoon K. Bioorg. Med. Chem. Lett. 2011;21:3708. - PubMed
-
- Ghosh P. Kwon N. Y. Kim S. Han S. Lee S. H. An W. Mishra N. K. Han S. B. Kim I. S. Angew. Chem., Int. Ed. 2021;60:191. - PubMed
- Ghosh P. Kwon N. Y. Byun Y. Mishra N. K. Park J. S. Kim I. S. ACS Catal. 2022;12:15707.
LinkOut - more resources
Full Text Sources