An electrochemical sensor based on MWCNTs and a PCCN222 peroxidase-like nanocomposite for sensitive and selective kaempferol detection
- PMID: 40213623
- PMCID: PMC11983268
- DOI: 10.1039/d5ra01094b
An electrochemical sensor based on MWCNTs and a PCCN222 peroxidase-like nanocomposite for sensitive and selective kaempferol detection
Abstract
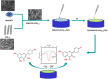
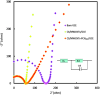
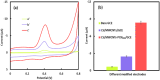
Kaempferol (KA) is a flavonoid with a range of biological properties, including antitumor, antioxidant, antiviral and anti-inflammatory, and its extensive applications in biomedicine, food safety, and related fields underscore the importance of quantitative analysis for determining its concentration. In this study, an electrochemical sensor based on multi-walled carbon nanotubes (MWCNTs), PCN222 and chitosan (CS) was developed for the determination of KA. MWCNTs exhibit hydrophobicity and conductivity, and they are better dispersed by DMF and crosslinked with PCN222, which further improves the electrode's response, selectivity and sensitivity to KA due to the peroxide-like properties of PCN222. The film formed by CS on the electrode surface serves to protect the nanocomposite from detaching during the operation. The linear range of this sensor is 0.01-0.4 and 0.6-9 μM, with a detection limit of 4.16 nM. This method can be used to detect the content of KA in plasma, which shows that the electrochemical sensor has strong practical application capabilities, as well as other advantages, such as high stability, strong anti-interference ability, and low detection limit. Moreover, the ultraviolet-visible spectrophotometer (UV) demonstrates that the catalytic rate of PCN222 for KA is significantly faster than that for naringenin and puerarin. Therefore, the construction of CS/MWCNT-PCN222/GCE electrochemical sensors has potential application value for clinical dosage control and monitoring of the drug metabolism of KA.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Nejabati H. R. Roshangar L. J. Food Biochem. 2022;46:e14375. - PubMed
LinkOut - more resources
Full Text Sources

