Drugless peptide-based nanohybrids alleviate diabetic retinopathy by suppressing microglial activation and endothelial inflammation
- PMID: 40213650
- PMCID: PMC11980661
- DOI: 10.7150/thno.102775
Drugless peptide-based nanohybrids alleviate diabetic retinopathy by suppressing microglial activation and endothelial inflammation
Abstract
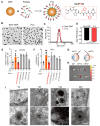
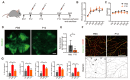
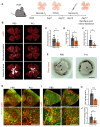
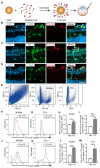
Background: Diabetic retinopathy (DR) is a vision-threatening microvascular complication of diabetes mellitus. Chronic inflammation and endothelial dysfunction are critical factors in the disease's pathogenesis. Consequently, interventions developed to reduce retinal inflammation are anticipated to be beneficial for both the prevention and treatment of DR. In the present study, we developed a unique class of drugless peptide-based nanohybrids with potent anti-inflammatory activities and investigated their therapeutic efficacy for treating DR in an oxygen-induced retinopathy (OIR) mouse model and a streptozotocin (STZ)-induced diabetic mouse model. Methods: Hexapeptides were applied to modify gold nanoparticles to form the drugless peptide-based nanohybrids (P12). We then examined the physicochemical properties and anti-inflammatory activities of P12 in HUVECs and BV2 cells and identified the critical amino acids for this novel bioactivity. The intravitreal and retro-orbital injections were applied to determine the optimal retinal delivery route for P12. The therapeutic efficacy of P12 in treating DR were investigated using both the OIR model and STZ-induced diabetic model. Through immunohistochemistry and flow cytometry analyses, we identified the major cells that internalize P12 in the retina. Furthermore, in vitro experiments were used to explore the underlying molecular mechanisms for the anti-inflammatory activities of P12. Results: We found that P12 exhibited potent anti-inflammatory effects in both HUVECs and BV2 cells. In addition, P12 can be efficiently delivered to the retina via intravitreal injection. Intravitreally injected P12 significantly improved early DR symptoms including vascular leakage and pericyte loss in STZ-induced diabetic mice. It also suppressed pathological neovascularization and retinal hemorrhage in OIR mice. Importantly, we found that intravitreally injected P12 was mainly taken up by microglial and endothelial cells, leading to reduced retinal endothelium inflammation and microglial activation in DR animal models. Mechanistic studies revealed that P12 potently inhibited several TLR4 downstream signaling pathways, such as NF-κB, JNK, and P38 MAPK, in both endothelial and microglial cells. This effect is due to the capacity of P12 in blocking the endosomal acidification process that governs the endosomal TLR signaling transduction. Conclusions: Our findings suggest that local injection of properly designed, drugless, peptide-based nanohybrids can serve as a safe and effective anti-inflammatory nanomedicine for treating DR.
Keywords: Toll-like receptors; bioactive nanoparticles; diabetic retinopathy; endothelial inflammation; microglia activation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation.Mol Vis. 2010 Oct 8;16:2033-42. Mol Vis. 2010. PMID: 21042558 Free PMC article.
-
Sesamin suppresses high glucose-induced microglial inflammation in the retina in vitro and in vivo.J Neurophysiol. 2022 Feb 1;127(2):405-411. doi: 10.1152/jn.00466.2021. Epub 2022 Jan 12. J Neurophysiol. 2022. PMID: 35020533
-
Size-dependent anti-inflammatory activity of a peptide-gold nanoparticle hybrid in vitro and in a mouse model of acute lung injury.Acta Biomater. 2019 Feb;85:203-217. doi: 10.1016/j.actbio.2018.12.046. Epub 2018 Dec 28. Acta Biomater. 2019. PMID: 30597258 Free PMC article.
-
Modulation of microglia in the retina: new insights into diabetic retinopathy.Acta Diabetol. 2017 Jun;54(6):527-533. doi: 10.1007/s00592-017-0984-z. Epub 2017 Mar 27. Acta Diabetol. 2017. PMID: 28349217 Review.
-
Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy.Prog Retin Eye Res. 2012 Sep;31(5):481-94. doi: 10.1016/j.preteyeres.2012.04.005. Epub 2012 May 15. Prog Retin Eye Res. 2012. PMID: 22609081 Free PMC article. Review.
References
-
- Ogurtsova K, Fernandes JDDR, Huang Y, Linnenkamp U, Guariguata L, Cho NH. et al. Idf diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. - PubMed
-
- Cho NH, Shaw JE, Karuranga S, Huang Y, Fernandes JDD, Ohlrogge AW. et al. Idf diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. - PubMed
-
- Tan GS, Cheung N, Simó R, Cheung GCM, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5:143–55. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

