A self-healing radiopaque hyaluronic acid hydrogel as a new injectable biomaterial for precision medicine in osteoarthritis
- PMID: 40213675
- PMCID: PMC11980665
- DOI: 10.7150/thno.104551
A self-healing radiopaque hyaluronic acid hydrogel as a new injectable biomaterial for precision medicine in osteoarthritis
Abstract
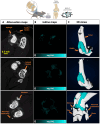
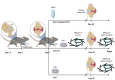
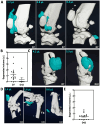
Rationale: Osteoarthritis (OA) is a degenerative disease affecting cartilage, synovium and bone, that is a major cause of pain and disability. Intra-articular injection of hyaluronic acid (HA) derivatives, also known as viscosupplementation (VS), is a common treatment for the symptomatic management of knee OA. Despite its widespread use, the magnitude of the clinical benefit of VS remains controversial, with conflicting results due to methodological differences and possible differences in efficacy between products related to remanence and rheological properties. Methods: Here, to create an effective HA-based treatment, an injectable self-healing HA hydrogel with long-persistent radiopacity is formed by tethering a clinical iodine contrast agent to HA. The labeling conditions are tuned to obtain sufficient X-ray signal without altering the biocompatibility, rheological and injectability properties of the hydrogel. Results: The iodine labeling enabled to monitor not only delivery of the hydrogel but also its retention in mouse knees up to 5 weeks post-administration using synchrotron K-edge subtraction-computed tomography. We further demonstrated that the unique properties of this hydrogel enable creation of a transient HA network in vivo that attenuates OA progression in a mouse model of OA. Moreover, our data showed that the rate of HA-I disappearance appears to predict treatment response, likely because a rapid elimination serves as an indirect indicator of in situ inflammation. Conclusion: Collectively, these results show that our radiopaque HA-I hydrogel holds significant promise for improving patient management in the treatment of OA before clinical symptoms worsen. Its capacity for in vivo tracking over time allows for personalized treatment schedules based on observed retention and therapeutic effect. As a result, this theranostic hydrogel emerges as a strong candidate for precision medicine in OA.
Keywords: Injectable hydrogel; Iodine; X-ray; hyaluronic acid; viscosupplementation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Gonçalves C, Carvalho DN, Silva TH, Reis RL, Oliveira JM. Engineering of viscosupplement biomaterials for treatment of osteoarthritis: A comprehensive review. Adv Eng Mater. 2022;24:2101541.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

