Human umbilical cord mesenchymal stem cells improve the pregnancy outcomes of preeclampsia rats via inducing placental angiogenesis
- PMID: 40213878
- PMCID: PMC12144550
- DOI: 10.1097/HJH.0000000000004022
Human umbilical cord mesenchymal stem cells improve the pregnancy outcomes of preeclampsia rats via inducing placental angiogenesis
Abstract
Background: Preeclampsia is considered to be a serious complication unique to pregnancy caused by placental dysplasia. Although interventions such as antihypertensive drugs and magnesium sulfate can partially mitigate maternal risk, the ultimate remedy remains delivery. In recent years, mesenchymal stem cells (MSCs) have become promising therapeutic approach in the treatment of ischemic diseases. Therefore, this study aimed to investigate the effect of human umbilical cord mesenchymal stem cells (hucMSCs) on pregnancy outcomes and placental angiogenesis in N-nitro-L-arginine methyl ester (L-NAME) induced preeclampsia rats.
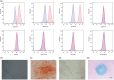
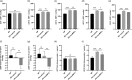
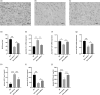
Methods: The expression of mesenchymal markers in hucMSCs was analyzed by flow cytometry. Multipotent differentiation of hucMSCs was identified, respectively. HucMSCs were injected into the preeclampsia rat by the tail vein. SBP was measured at the rat tail artery using an automatic noninvasive blood pressure monitor. The proteinuria levels were measured using the BCA method. RT-qPCR and ELISA were used to assess the mRNA expression and plasma concentrations of soluble FMS-like tyrosine kinase 1 (sFlt-1), placental growth factor (PLGF), and vascular endothelial growth factor (VEGF). Placental tissues were collected for immunohistochemistry and pathological analysis.
Results: HucMSCs were positive for CD90, CD73, and CD105, and could differentiate into osteoblasts, adipocytes and chondrocytes. PE rats treated with hucMSCs showed a lowering of SBP and proteinuria and a higher fetal and placental mass. The microvascular density (MVD), mRNA expression, and plasma concentrations of VEGF and PLGF were increased in the hucMSCs-treated group, while the sFlt-1 levels were decreased.
Conclusion: HucMSCs may promote placental angiogenesis and improve the pregnancy outcomes of preeclampsia rats by regulating the balance of pro-angiogenic and antiangiogenic factors.
Keywords: angiogenesis; human umbilical cord mesenchymal stem cells; placental vasculature; preeclampsia; pregnancy outcomes.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures



Similar articles
-
Protective effect of human umbilical cord mesenchymal stem cell exosomes on preserving the morphology and angiogenesis of placenta in rats with preeclampsia.Biomed Pharmacother. 2018 Sep;105:1240-1247. doi: 10.1016/j.biopha.2018.06.032. Epub 2018 Jun 22. Biomed Pharmacother. 2018. PMID: 30021360
-
The Protective Benefit of Heme Oxygenase-1 Gene-Modified Human Placenta-Derived Mesenchymal Stem Cells in a N-Nitro-L-Arginine Methyl Ester-Induced Preeclampsia-Like Rat Model: Possible Implications for Placental Angiogenesis.Stem Cells Dev. 2021 Oct 1;30(19):991-1002. doi: 10.1089/scd.2021.0174. Stem Cells Dev. 2021. PMID: 34470469
-
Human umbilical cord mesenchymal stem cell derived exosomes (HUCMSC-exos) recovery soluble fms-like tyrosine kinase-1 (sFlt-1)-induced endothelial dysfunction in preeclampsia.Eur J Med Res. 2023 Aug 9;28(1):277. doi: 10.1186/s40001-023-01182-8. Eur J Med Res. 2023. PMID: 37559150 Free PMC article.
-
Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome.Hypertension. 2020 Apr;75(4):918-926. doi: 10.1161/HYPERTENSIONAHA.119.13763. Epub 2020 Feb 17. Hypertension. 2020. PMID: 32063058 Free PMC article. Review.
-
[Potential value of placental angiogenic factors as biomarkers in preeclampsia for clinical physicians].Nephrol Ther. 2019 Nov;15(6):413-429. doi: 10.1016/j.nephro.2018.10.005. Epub 2019 Mar 30. Nephrol Ther. 2019. PMID: 30935786 Review. French.
References
-
- Gestational hypertension and preeclampsia: Acog practice bulletin, number 222. Obstet Gynecol 2020; 135:e237–e260. - PubMed
-
- Magee LA, Nicolaides KH, Von Dadelszen P. Preeclampsia. N Engl J Med 2022; 386:1817–1832. - PubMed
-
- Tong S, Kaitu’u-Lino TJ, Hastie R, Brownfoot F, Cluver C, Hannan N. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia. Am J Obstet Gynecol 2022; 226:S1157–S1170. - PubMed
-
- Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-pathophysiology and clinical presentations: Jacc state-of-the-art review. J Am Coll Cardiol 2020; 76:1690–1702. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

