Feasibility of MRI-Guided Transperineal Implantation of Microdevices for Drug Delivery and Response Assessment in Prostate Cancer
- PMID: 40213929
- PMCID: PMC12335932
- DOI: 10.1002/jmri.29784
Feasibility of MRI-Guided Transperineal Implantation of Microdevices for Drug Delivery and Response Assessment in Prostate Cancer
Abstract
Background: Prostate cancer (PCa) treatment often involves systemic therapies with varying mechanisms of action, affecting individuals differently. Implantable microdevices (IMDs) are designed to test multiple drugs within a patient's tumor, but the feasibility of MRI-guided placement in PCa has not been evaluated.
Purpose: To provide proof of concept for placing IMDs into lesions with MRI guidance to predict patient-specific responses to therapies.
Study type: Prospective.
Population: Fifteen participants undergoing prostatectomy for PCa.
Field strength/sequence: 3T MRI With T2-weighted (T2W).
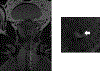
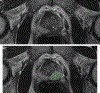
Assessment: In-bore MRI-targeted placement of IMDs was performed. Intra-procedural MRI scans were reviewed by a radiologist, using needle artifacts on T2W images to guide IMD placement. A genitourinary pathologist performed Gleason scoring around the IMDs. Drug response analysis included Enzalutamide + Nivolumab, Enzalutamide + Docetaxel, and single-agent Enzalutamide.
Statistical tests: Mann-Whitney U test for continuous variables, p < 0.05 for significance.
Results: Of 53 IMDs implanted into suspicious lesions in 14 participants, 48 (90%) were successfully placed within the lesions. The average distance from the needle tip to the tumor was 8.32 ± 4.02 mm. Larger lesion size (p = 0.009) and lower prostate imaging-reporting and data system score (p = 0.031) were significantly associated with successful IMD placement. Of the 53 IMDs, 49 (92.4%) were retrieved for histopathology and drug response analysis. In four participants, Gleason scores around the device were lower than preplacement biopsy in two and equal in two. Additionally, drug analysis in one patient demonstrated the feasibility of drug response analysis, revealing differences in apoptotic index, lymphocyte infiltration, dysplastic cell composition, and cellular profiles for each treatment. No complications or adverse events occurred.
Conclusion: IMDs can be effectively and safely placed in prostate lesions using MRI guidance, with feasible histological and drug response analyses.
Evidence level: 2. Technical Efficacy: Stage 1.
Keywords: MRI; interventional radiology; microdevice; prostate cancer.
© 2025 International Society for Magnetic Resonance in Medicine.
Figures

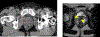


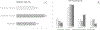
Similar articles
-
Novel Intraprostatic Magnetic Resonance-Guided Implantation of Multidrug-Eluting Microdevice for Testing of Systemic Therapy Agents In Situ: Proof of Concept in Intermediate-Risk and High-Risk Prostate Cancer.J Urol. 2025 Feb;213(2):173-182. doi: 10.1097/JU.0000000000004269. Epub 2024 Sep 30. J Urol. 2025. PMID: 39348711 Clinical Trial.
-
Histopathologic Yields and Concordance of In-Bore MRI-targeted Biopsy for Prostate Cancer Diagnosis.Radiology. 2025 Jul;316(1):e241710. doi: 10.1148/radiol.241710. Radiology. 2025. PMID: 40728405
-
Clinical feasibility of MRI-guided in-bore prostate biopsies at 0.55T.Abdom Radiol (NY). 2025 Aug;50(8):3773-3783. doi: 10.1007/s00261-024-04783-x. Epub 2025 Jan 11. Abdom Radiol (NY). 2025. PMID: 39794536
-
MRI software and cognitive fusion biopsies in people with suspected prostate cancer: a systematic review, network meta-analysis and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(61):1-310. doi: 10.3310/PLFG4210. Health Technol Assess. 2024. PMID: 39367754 Free PMC article.
-
The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation.Health Technol Assess. 2013 May;17(20):vii-xix, 1-281. doi: 10.3310/hta17200. Health Technol Assess. 2013. PMID: 23697373 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

