Functional Pulmonary Imaging
- PMID: 40213976
- PMCID: PMC12435115
- DOI: 10.1002/jmri.29778
Functional Pulmonary Imaging
Abstract
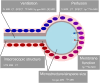
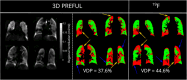
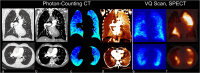
The aging of the world population gave rise to an increased prevalence of many lung diseases, with chronic obstructive pulmonary disease now ranking as the third-leading cause of death according to the World Health Organization. To diagnose lung disease, a thorough assessment of lung function is essential since it may reveal unique signatures in terms of disease pathophysiology. Yet, clinically established lung function tests are global measurements, which may compromise their sensitivity to early, regional changes in lung function compared to spatially resolved imaging tests. From a scientific perspective, the lung is a highly complex organ, and newly developed functional imaging methods may elucidate previously unknown aspects of its physiology. Functional pulmonary imaging is and will thus be of great value for both clinical and research applications. The goal of this review is to shed light on the field of functional pulmonary imaging in all its varieties, with a particular focus on the numerous tools MRI has to offer. This includes 1H MRI methods with or without exogenous contrast agents like oxygen- or gadolinium-based contrast agents and MRI of hyperpolarized and inert gases like 129Xe or perfluoropropane. However, thinking outside the box, a glance is also taken at what other modalities like single-photon emission computed tomography, computed tomography, or X-ray dark-field imaging have to offer. Following a physiological perspective, methods are described in terms of their ability to assess the key parameters of lung physiology in humans-ventilation, perfusion, and alveolar membrane function, as well as microstructure-and promising clinical and research applications are discussed. An outlook into possible future paths the field might take is given. Evidence Level: 5. Technical Efficacy: 2.
Keywords: alveolar membrane function; imaging; lung function; microstructure; perfusion; ventilation.
© 2025 The Author(s). Journal of Magnetic Resonance Imaging published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures

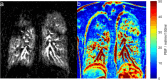
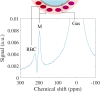


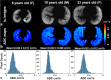
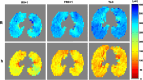
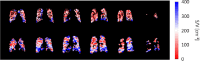
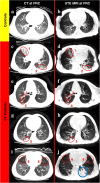
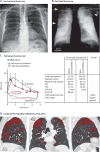
References
-
- World Health Organization , “The Top 10 Causes of Death,” (2020), https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death.
-
- Gehr P., Bachofen M., and Weibel E. R., “The Normal Human Lung: Ultrastructure and Morphometric Estimation of Diffusion Capacity,” Respiration Physiology 32 (1978): 121–140. - PubMed
-
- Grote J., “Die Sauerstoffdiffusionskonstanten im Lungengewebe und Wasser und ihre Temperaturabhängigkeit,” Pflügers Archiv für die Gesamte Physiologie des Menschen und der Tiere 295 (1967): 245–254. - PubMed
-
- Kauczor H.‐U., Ebert M., Kreitner K.‐F., et al., “Imaging of the Lungs Using 3He MRI: Preliminary Clinical Experience in 18 Patients With and Without Lung Disease,” Journal of Magnetic Resonance Imaging 7 (1997): 538–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

