KCS1 and VIP1, the genes encoding yeast phosphoinositol pyrophosphate synthases, are required for Ca2+-mediated response to dimethylsulfoxide (DMSO)
- PMID: 40214101
- PMCID: PMC12226410
- DOI: 10.1002/2211-5463.70039
KCS1 and VIP1, the genes encoding yeast phosphoinositol pyrophosphate synthases, are required for Ca2+-mediated response to dimethylsulfoxide (DMSO)
Abstract
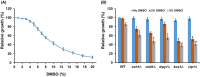
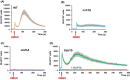
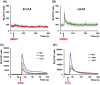
Dimethylsulfoxide (DMSO) is widely used as a solvent or as a carrier when screening for biologic activity of various chemicals, but results need to be interpreted carefully due to its intrinsic toxicity. DMSO has been previously observed to impair the growth of yeast cells defective in calcium movement across cellular membranes and in phosphoinositol pyrophosphate synthases. Here, we set out to investigate the Ca2+-mediated response to DMSO in Saccharomyces cerevisiae. The cell exposure to DMSO was signaled by a two-phase cytosolic Ca2+ wave that was dependent on Mid1, a subunit of the Cch1/Mid1 Ca2+ channel located at the plasma membrane. While the vacuolar Ca2+ channel Trpy1 also contributed by releasing Ca2+ from the vacuole, the immediate cell response to DMSO exposure depended on the external Ca2+ imported into the cell through Cch1/Mid1. A chemogenomic screen previously performed on a collection of yeast knockout mutants identified the two phosphoinositol pyrophosphate synthases Kcs1 and Vip1 as determinants for yeast tolerance to DMSO. Deletion of KCS1 or VIP1 genes suppressed the DMSO-induced Ca2+ response, suggesting that both Ca2+ and phosphoinositol pyrophosphate signaling contribute to cell adaptation under DMSO stress.
Keywords: Saccharomyces cerevisiae; aequorin; calcium; dimethylsulfoxide; inositol pyrophosphate synthase.
© 2025 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

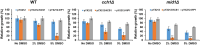
Similar articles
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Di L and Kerns EH (2006) Biological assay challenges from compound solubility: strategies for bioassay optimization. Drug Discov Today 11, 446–451. - PubMed
-
- Rammler DH and Zaffaroni A (1967) Biological implications of DMSO based on a review of its chemical properties. Ann N Y Acad Sci 141, 13–23. - PubMed
-
- Sahu S, Garg A, Saini R and Debnath A (2024) Interface water assists in dimethyl sulfoxide crossing and poration in model bilayer. Langmuir 40, 5764–5775. - PubMed
-
- Matias M, Silvestre S, Falcão A and Alves G (2018) Considerations and pitfalls in selecting the drug vehicles for evaluation of new drug candidates: focus on in vivo pharmaco‐toxicological assays based on the rotarod performance test. J Pharm Pharm Sci 21, 110–118. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

