UTERINE FIBROIDS
- PMID: 40214304
- PMCID: PMC12419501
- DOI: 10.1152/physrev.00010.2024
UTERINE FIBROIDS
Abstract
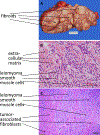
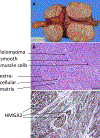
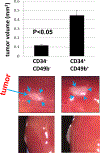
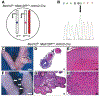
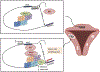
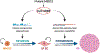
Uterine fibroids (leiomyomas), the most common tumors in women and those assigned female at birth, originate from myometrial smooth muscle cells and cause heavy menstrual bleeding, anemia, pelvic discomfort, pregnancy loss, and obstruction of labor in approximately a quarter of reproductive-age women. During each ovulatory cycle, the myometrium responds to the ovarian steroids, estradiol, and progesterone, via increased tissue stem cell proliferation to prepare for impending pregnancy, during which a somatic mutation may arise to initiate a tumor. Both the mutated smooth muscle cells and the adjacent tumor-associated fibroblasts lay down excessive quantities of extracellular matrix, providing a unique feature that led to naming these tumors "fibroids." The most common somatic mutations in fibroids affect the Mediator complex subunit 12 (MED12; 77%) and high-mobility group AT-hook 2/1 (HMGA2/1; 10%) genes. Heterozygous mutations in MED12, a chromatin-associated protein, disrupt the attached CDK8 kinase module in the Mediator complex. MED12 mutations are associated with increased genomic instability, altered chromatin landscape and enhancer engagement, and increased responsiveness to progesterone. Progesterone and a small stem cell population in a fibroid are essential for tumor survival and growth. Progesterone, via its receptors in differentiated fibroid cell populations, activates the production of Wingless-type MMTV integration site family (WNT) ligands, cytokines, and other growth substances to act on adjacent stem cells in a paracrine fashion to support tumor growth. Suppression of estrogen or progesterone production and progesterone antagonists have been used for temporary shrinkage of these tumors and symptom relief. Here we provide an overview of these mechanisms and future approaches for prevention and medical management of uterine fibroids.
Keywords: HMGA2; MED12; progesterone; stem cell; uterine fibroid.
Figures




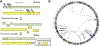
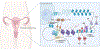







References
-
- Day Baird D, Dunson DB, Hill MC, Cousins D, and Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 188: 100–107, 2003. - PubMed
-
- Farquhar CM, and Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol 99: 229–234, 2002. - PubMed
-
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, and Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2: 16043, 2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HD087417/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- R01 CA298456/CA/NCI NIH HHS/United States
- R01 ES034753/ES/NIEHS NIH HHS/United States
- R01ES034753/HHS | NIH | National Institute of Environmental Health Sciences (DEHS)
- P50HD098580/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources
Medical

