Cell Progression and Survival Functions of Enzymes Secreted in Extracellular Vesicles Associated with Breast and Prostate Cancers
- PMID: 40214422
- PMCID: PMC11988166
- DOI: 10.3390/cells14070468
Cell Progression and Survival Functions of Enzymes Secreted in Extracellular Vesicles Associated with Breast and Prostate Cancers
Abstract
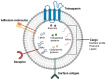
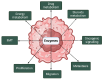
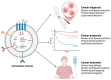
Extracellular vesicles (EVs) are membrane-bound cargoes secreted by normal and pathological cells. Through their protein, nucleic acid, and lipid cargoes, EVs mediate several cellular processes, such as cell-cell communication, cell development, immune response, and tissue repair. Most importantly, through their enzyme cargo, EVs mediate pathophysiological processes, including the pathogenesis of cancer. In this review, we enumerate several enzymes secreted in EVs (EV enzyme cargo) from cells and patient clinical samples of breast and prostate cancers and detail their contributions to the progression and survival of both cancers. Findings in this review reveal that the EV enzyme cargo could exert cell progression functions via adhesion, proliferation, migration, invasion, and metastasis. The EV enzyme cargo might also influence cell survival functions of chemoresistance, radioresistance, angiogenesis, cell death inhibition, cell colony formation, and immune evasion. While the current literature provides evidence of the possible contributions of the EV enzyme cargo to the progression and survival mechanisms of breast and prostate cancers, future studies are required to validate that these effects are modified by EVs and provide insights into the clinical applications of the EV enzyme cargo in breast and prostate cancer.
Keywords: breast; cancer; enzymes; extracellular; progression; prostate; proteins; survival; tumor; vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Extracellular Vesicles from 3D Engineered Microtissues Harbor Disease-Related Cargo Absent in EVs from 2D Cultures.Adv Healthc Mater. 2022 Mar;11(5):e2002067. doi: 10.1002/adhm.202002067. Epub 2021 Apr 22. Adv Healthc Mater. 2022. PMID: 33890421
-
Extracellular vesicles in the treatment and diagnosis of breast cancer: a status update.Front Endocrinol (Lausanne). 2023 Jul 17;14:1202493. doi: 10.3389/fendo.2023.1202493. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37534210 Free PMC article. Review.
-
CD81-guided heterologous EVs present heterogeneous interactions with breast cancer cells.J Biomed Sci. 2024 Oct 15;31(1):92. doi: 10.1186/s12929-024-01084-9. J Biomed Sci. 2024. PMID: 39402557 Free PMC article.
-
Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1.Cancer Gene Ther. 2021 Jun;28(6):634-648. doi: 10.1038/s41417-020-00244-x. Epub 2020 Nov 5. Cancer Gene Ther. 2021. PMID: 33154575
-
Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation.Semin Cell Dev Biol. 2017 Jul;67:11-22. doi: 10.1016/j.semcdb.2017.01.003. Epub 2017 Jan 8. Semin Cell Dev Biol. 2017. PMID: 28077296 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

