Imeglimin Inhibits Macrophage Foam Cell Formation and Atherosclerosis in Streptozotocin-Induced Diabetic ApoE-Deficient Mice
- PMID: 40214426
- PMCID: PMC11988081
- DOI: 10.3390/cells14070472
Imeglimin Inhibits Macrophage Foam Cell Formation and Atherosclerosis in Streptozotocin-Induced Diabetic ApoE-Deficient Mice
Abstract
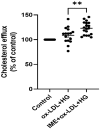
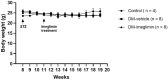
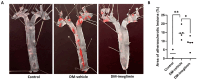
Atherosclerotic cardiovascular disease is a major complication of diabetes, whose progression is significantly accelerated by hyperglycemia. Imeglimin, a novel oral antidiabetic agent, has demonstrated efficacy in glucose control; however, its role in diabetes-related cardiovascular complications has not yet been fully explored. This study aimed to investigate the effects of imeglimin on foam cell formation and atherosclerosis in the context of diabetes. THP-1 macrophages were treated with oxidized low-density lipoprotein (LDL) and high glucose to induce foam cell formation in vitro. Additionally, ApoE-/- mice with streptozotocin-induced diabetes were used to determine the effects of imeglimin in vivo by analyzing metabolic parameters and atherosclerotic plaque formation. Imeglimin inhibited macrophage-derived foam cell formation by promoting the expression of ATP-binding cassette transporters (ABC) A1 and ABCG1 and downregulating the expression of CD36. The effects of imeglimin on ABCG1 and CD36 expression regulation was mediated by AMPK. In diabetic ApoE-/- mice, imeglimin reduced the atherosclerotic plaque area, decreased fasting glucose and LDL cholesterol levels, and upregulated ABCG1 expression in the liver and aorta. These findings suggest that imeglimin may have a preventive effect on foam cell formation and a therapeutic role in atherosclerosis progression in diabetic conditions.
Keywords: atherosclerosis; cholesterol efflux; foam cell formation; imeglimin; macrophage; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Han E., Song S.O., Kim H.S., Son K.J., Jee S.H., Cha B.S., Lee B.W. Improvement in Age at Mortality and Changes in Causes of Death in the Population with Diabetes: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018. Endocrinol. Metab. 2022;37:466–474. doi: 10.3803/EnM.2022.1440. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

