Opposite Roles of IL-32α Versus IL-32β/γ Isoforms in Promoting Monocyte-Derived Osteoblast/Osteoclast Differentiation and Vascular Calcification in People with HIV
- PMID: 40214435
- PMCID: PMC11987946
- DOI: 10.3390/cells14070481
Opposite Roles of IL-32α Versus IL-32β/γ Isoforms in Promoting Monocyte-Derived Osteoblast/Osteoclast Differentiation and Vascular Calcification in People with HIV
Abstract
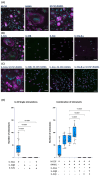
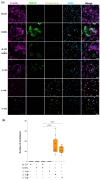
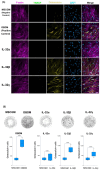
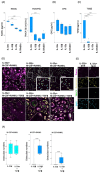
People with HIV (PWH) have an increased risk of developing cardiovascular disease (CVD). Our recent data demonstrated that the multi-isoform proinflammatory cytokine IL-32 is upregulated in PWH and is associated with arterial stiffness and subclinical atherosclerosis. However, the mechanisms by which IL-32 contributes to the pathogenesis of these diseases remain unclear. Here, we show that while the less expressed IL-32α isoform induces the differentiation of human classical monocytes into the calcium-resorbing osteoclast cells, the dominantly expressed isoforms IL-32β and IL-32γ suppress this function through the inhibition of TGF-β and induce the differentiation of monocytes into the calcium-depositing osteocalcin+ osteoblasts. These results aligned with the increase in plasma levels of osteoprotegerin, a biomarker of vascular calcification, and its association with the presence of coronary artery subclinical atherosclerosis and calcium score in PWH. These findings support a novel role for the proinflammatory cytokine IL-32 in the pathophysiology of CVD by increasing vascular calcification in PWH.
Keywords: HIV; IL-32; TGF-β; arterial calcification; atherosclerosis; cardiovascular diseases; inflammation; osteoblasts; osteoclasts; osteoprotegerin.
Conflict of interest statement
J.R.K. reports stock ownership in Abbott, AbbVie, Bristol Myers Squibb, Johnson & Johnson, Eli Lilly, Medtronic, Merck, and Pfizer.
Figures
References
-
- Guzman-Fulgencio M., Medrano J., Rallon N., Echeverria-Urabayen A., Miguel Benito J., Restrepo C., Garcia-Alvarez M., Vispo E., San Roman J., Sanchez-Piedra C., et al. Soluble markers of inflammation are associated with Framingham scores in HIV-infected patients on suppressive antiretroviral therapy. J. Infect. 2011;63:382–390. doi: 10.1016/j.jinf.2011.08.006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

