The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics
- PMID: 40214455
- PMCID: PMC11988096
- DOI: 10.3390/cells14070501
The Impact of TRPM8 on Prostate Cancer Transcriptomic Dynamics
Abstract
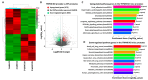
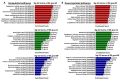
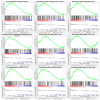
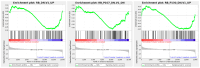
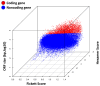
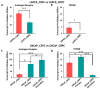
Prostate cancer (PC) remains a significant health challenge, with androgen receptor (AR) signaling playing a pivotal role in its progression. This study investigates the expression and functional implications of the transient receptor potential melastatin 8 (TRPM8) channel in PC, focusing on its interaction with AR and its impact on oncogenic pathways. We analyzed mRNA expression levels of TRPM8 and AR in PC tissues, revealing that TRPM8 is upregulated in benign and early-stage tumors but significantly downregulated in metastatic samples. This decline correlates with increased AR expression, suggesting a compensatory mechanism that enhances AR-driven tumorigenesis. RNA sequencing and pathway enrichment analyses demonstrated that TRPM8 knockout (KO) prostates exhibited significant alterations in gene expression, particularly in pathways related to extracellular matrix (ECM) remodeling, cell proliferation, and survival signaling. Notably, genes associated with metastasis, such as MMP2 and FAP, were upregulated in TRPM8 KO samples, indicating a potential role for TRPM8 in inhibiting tumor invasion. Furthermore, Gene Set Enrichment Analysis (GSEA) revealed positive enrichment of androgen response, angiogenesis, and epithelial-mesenchymal transition (EMT) pathways in TRPM8 KO prostates, reinforcing the notion that TRPM8 loss creates a pro-tumorigenic environment. Our findings suggest that TRPM8 functions as a molecular brake on PC progression, and its loss may contribute to the development of aggressive disease phenotypes. This study underscores the importance of TRPM8 as a potential therapeutic target and biomarker in PC, warranting further investigation into its role in cancer biology and treatment response.
Keywords: TRPM8; androgen; androgen receptor (AR); mRNA expression; prostate cancer.
Conflict of interest statement
Jai Velpula volunteers at Pringle Robotics, learning the skill sets for analysis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Yanagisawa T., Rajwa P., Quhal F., Kawada T., Bekku K., Laukhtina E., von Deimling M., Chlosta M., Karakiewicz P.I., Kimura T., et al. Neoadjuvant androgen receptor signaling inhibitors before radical prostatectomy for non-metastatic advanced prostate cancer: A systematic review. J. Pers. Med. 2023;13:641. doi: 10.3390/jpm13040641. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

