Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors
- PMID: 40214464
- PMCID: PMC11987926
- DOI: 10.3390/cells14070510
Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors
Abstract
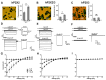
P2X receptors are unspecific cation channels activated by ATP. They are expressed in various tissues and found in neuronal and immune cells. In mammals, seven subunits are described, which can assemble into homomeric and heteromeric trimers. P2X2 receptors play important roles in cochlear adaptation to elevated sound levels. Three mutations causing inherited progressive hearing loss have been identified. These mutations localize to the transmembrane domain 1 (V60L), the transmembrane domain 2 (G353R) and a β-sheet linking the ATP binding site to the pore (D273Y). Herein, mutations were studied in human homomeric P2X2 as well as in heteromeric P2X2/3 receptors. We measured their binding of a fluorescently labeled ATP derivative (fATP) and characterized the constructs using the patch-clamp technique. The conclusions from our results are as follows: 1. The mutations V60L and G353R show robust localization on the plasma membrane and binding of fATP, whereas the mutant D273Y has no binding to fATP. 2. The mutation V60L has an increased affinity to fATP compared with the wildtype. 3. The expression of hP2X2 V60L channels reduces cell viability, which may support its role in the pathogenesis of hearing loss. 4. All mutant P2X2 subunits can assemble into P2X2/3 heteromeric channels with distinct phenotypes.
Keywords: P2X receptors; fluorescent ATP; hearing loss mutations; heteromeric ion channels; ligand-binding assay; patch-clamp.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Hearing loss mutations alter the functional properties of human P2X2 receptor channels through distinct mechanisms.Proc Natl Acad Sci U S A. 2019 Nov 5;116(45):22862-22871. doi: 10.1073/pnas.1912156116. Epub 2019 Oct 21. Proc Natl Acad Sci U S A. 2019. PMID: 31636190 Free PMC article.
-
Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors.Biomolecules. 2024 Aug 2;14(8):942. doi: 10.3390/biom14080942. Biomolecules. 2024. PMID: 39199330 Free PMC article.
-
Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):E3455-63. doi: 10.1073/pnas.1308088110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959888 Free PMC article.
-
Pharmacology of cloned P2X receptors.Annu Rev Pharmacol Toxicol. 2000;40:563-80. doi: 10.1146/annurev.pharmtox.40.1.563. Annu Rev Pharmacol Toxicol. 2000. PMID: 10836147 Review.
-
[Structual and functional dynamics of the ATP receptor channel P2X(2)].Brain Nerve. 2010 Dec;62(12):1323-9. Brain Nerve. 2010. PMID: 21139185 Review. Japanese.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

