Long-Term Impairment of Retinal Ganglion Cell Function After Oxygen-Induced Retinopathy
- PMID: 40214465
- PMCID: PMC11988018
- DOI: 10.3390/cells14070512
Long-Term Impairment of Retinal Ganglion Cell Function After Oxygen-Induced Retinopathy
Abstract
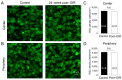
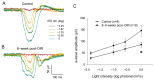
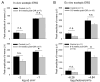
Premature infants with retinopathy of prematurity (ROP) have neovascularization of the retina, potentially resulting in low vision and even blindness. Some of these infants still have visual impairment, even if ROP resolves as they age. However, the mechanisms underlying the visual problems post-ROP are poorly understood. Because the pathological neovascularization in ROP infants can be mimicked in a mouse model with oxygen-induced retinopathy (OIR), we recapitulated post-ROP with post-OIR mice a few months after spontaneous regression of retinal neovascularization. Our pattern electroretinogram test demonstrates that post-OIR mice exhibit reduced P1-N2 responses, suggesting the impairment of retinal ganglion cells, the retina's output neurons. However, immunohistochemistry reveals that the density of retinal ganglion cells remains unchanged in post-OIR mice, indicating that the aforementioned pattern electroretinogram changes are functional. Our data further demonstrate that both light-adapted ex vivo electroretinogram a-waves (cone responses) and in vivo electroretinogram b-waves (ON cone bipolar cell responses) were significantly impaired in post-OIR mice. These results suggest that post-OIR impairment of the retinal cone pathway appears to result in the dysfunction of retinal ganglion cells, contributing to visual problems. A similar cellular mechanism could occur in post-ROP children, which is responsible for their visual impairment.
Keywords: bipolar cell; cone; oxygen–induced retinopathy; pattern electroretinogram; retinal ganglion cell; retinopathy of prematurity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

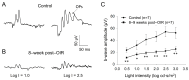

Similar articles
-
Long-term evaluation of retinal morphology and function in a mouse model of oxygen-induced retinopathy.Mol Vis. 2020 Apr 1;26:257-276. eCollection 2020. Mol Vis. 2020. PMID: 32256029 Free PMC article.
-
Simultaneous assessment of aberrant retinal vascularization, thickness, and function in an in vivo mouse oxygen-induced retinopathy model.Eye (Lond). 2019 Mar;33(3):363-373. doi: 10.1038/s41433-018-0205-1. Epub 2018 Sep 12. Eye (Lond). 2019. PMID: 30209267 Free PMC article.
-
IC100, a humanized therapeutic monoclonal anti-ASC antibody alleviates oxygen-induced retinopathy in mice.Angiogenesis. 2024 Aug;27(3):423-440. doi: 10.1007/s10456-024-09917-9. Epub 2024 May 6. Angiogenesis. 2024. PMID: 38709389 Free PMC article.
-
Retinal vascular development and oxygen-induced retinopathy: a role for adenosine.Prog Retin Eye Res. 2003 Jan;22(1):95-111. doi: 10.1016/s1350-9462(02)00058-7. Prog Retin Eye Res. 2003. PMID: 12597925 Review.
-
The progress of prophylactic treatment in retinopathy of prematurity.Int J Ophthalmol. 2018 May 18;11(5):858-873. doi: 10.18240/ijo.2018.05.24. eCollection 2018. Int J Ophthalmol. 2018. PMID: 29862189 Free PMC article. Review.
References
-
- Smith L.E., Wesolowski E., McLellan A., Kostyk S.K., D’Amato R., Sullivan R., D’Amore P.A. Oxygen–induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994;35:101–111. - PubMed
-
- Penn J.S., Tolman B.L., Henry M.M. Oxygen–induced retinopathy in the rat: Relationship of retinal nonperfusion to subsequent neovascularization. Investig. Ophthalmol. Vis. Sci. 1994;35:3429–3435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

