A Broad-Spectrum Chemokine Inhibitor Drives M2 Macrophage Polarization Through Modulation of the Myometrial Secretome
- PMID: 40214468
- PMCID: PMC11989072
- DOI: 10.3390/cells14070514
A Broad-Spectrum Chemokine Inhibitor Drives M2 Macrophage Polarization Through Modulation of the Myometrial Secretome
Abstract
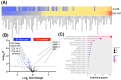
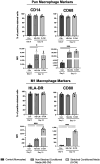
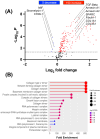
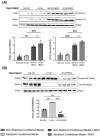
The uterine smooth muscle (myometrium) is an immunomodulatory tissue capable of secreting multiple chemokines during pregnancy. We propose that before term labor, chemokines secreted as a result of mechanical stretch of the uterine walls by the growing fetus(es) induce infiltration of maternal monocytes into myometrium, drive their differentiation into macrophages, and induce pro-inflammatory (M1) polarization, leading to labor contractions. This study used high-throughput proteomic mass-spectrometry to investigate the underlying mechanisms and explored the therapeutic potential of a broad-spectrum chemokine inhibitor (BSCI, FX125L) in modulating these effects. Primary myocytes isolated from the myometrium of term pregnant women were subjected in vitro to static mechanical stretch. Proteomic analysis of stretched myocyte-conditioned media (CM) identified significant upregulation of chemokine-related pathways and ECM degradation proteins. CM induced in vitro differentiation of human monocytes to macrophages and polarization into an M1-like phenotype characterized by elevated ROS production. BSCI treatment altered the myocyte secretome, increasing tissue-remodeling and anti-inflammatory proteins, Annexin A1 and TGF-β. BSCI-treated myocyte secretions induced Annexin A1 expression in macrophages and enhanced their phagocytic activity. We conclude that factors secreted by mechanically stretched myocytes induce pro-inflammatory M1 macrophage polarization, while BSCI modulates myocyte secretome, which reprograms macrophages to a homeostatic M2-like phenotype, thus reducing inflammation. When treated with BSCI, M2-polarized macrophages reduced myocyte-driven collagen gel contraction, whereas M1 macrophages enhanced it. This study reveals novel insights into the myocyte-macrophage interaction and identifies BSCI as a promising drug to modulate myometrial activity. We suggest that uterine macrophages may represent a therapeutic target for preventing preterm labor in women.
Keywords: broad spectrum chemokine inhibitor; cytokine; inflammation; labor; macrophage; mass-spectrometry; mechanical stretch; myometrium; pregnancy; uterus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
A Broad-Spectrum Chemokine Inhibitor Blocks Inflammation-Induced Myometrial Myocyte-Macrophage Crosstalk and Myometrial Contraction.Cells. 2021 Dec 31;11(1):128. doi: 10.3390/cells11010128. Cells. 2021. PMID: 35011690 Free PMC article.
-
Crosstalk between monocytes and myometrial smooth muscle in culture generates synergistic pro-inflammatory cytokine production and enhances myocyte contraction, with effects opposed by progesterone.Mol Hum Reprod. 2015 Aug;21(8):672-86. doi: 10.1093/molehr/gav027. Epub 2015 May 22. Mol Hum Reprod. 2015. PMID: 26002969 Free PMC article.
-
Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice.J Cell Mol Med. 2014 Sep;18(9):1816-29. doi: 10.1111/jcmm.12307. Epub 2014 Jun 4. J Cell Mol Med. 2014. PMID: 24894878 Free PMC article.
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
-
Myometrial activation: Novel concepts underlying labor.Placenta. 2020 Mar;92:28-36. doi: 10.1016/j.placenta.2020.02.005. Epub 2020 Feb 4. Placenta. 2020. PMID: 32056784 Review.
References
-
- Quinn J.-A., Munoz F.M., Gonik B., Frau L., Cutland C., Mallett-Moore T., Kissou A., Wittke F., Das M., Nunes T., et al. Preterm Birth: Case Definition & Guidelines for Data Collection, Analysis, and Presentation of Immunisation Safety Data. Vaccine. 2016;34:6047–6056. doi: 10.1016/j.vaccine.2016.03.045. - DOI - PMC - PubMed
-
- March of Dimes Report Card. [(accessed on 14 January 2025)]. Available online: https://www.marchofdimes.org/report-card.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

