Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium
- PMID: 40214475
- PMCID: PMC11988041
- DOI: 10.3390/cells14070522
Diverse Roles of the Multiple Phosphodiesterases in the Regulation of Cyclic Nucleotide Signaling in Dictyostelium
Abstract
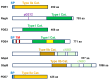
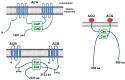
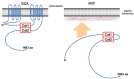
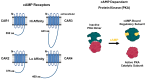
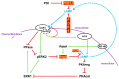
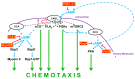
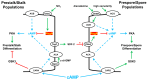
Dictyostelium is a unique model used to study the complex and interactive cyclic nucleotide signaling pathways that regulate multicellular development. Dictyostelium grow as individual single cells, but in the absence of nutrients, they initiate a multicellular developmental program. Central to this is secreted cAMP, a primary GPCR-response signal. Activated cAMP receptors at the cell surface direct a number of downstream signaling pathways, including synthesis of the intracellular second messengers cAMP and cGMP. These, in turn, activate a series of downstream targets that direct chemotaxis within extracellular cAMP gradients, multicellular aggregation, and, ultimately, cell-specific gene expression, morphogenesis, and cytodifferentiation. Extracellular cAMP and intracellular cAMP and cGMP exhibit rapid fluctuations in concentrations and are, thus, subject to exquisite regulation by both synthesis and degradation. The Dictyostelium genome encodes seven phosphodiesterases (PDEs) that degrade cyclic nucleotides to nucleotide 5'-monophosphates. Each PDE has a distinct structure, substrate specificity, regulatory input, cellular localization, and developmentally regulated expression pattern. The intra- or extra-cellular localizations and enzymatic specificities for cAMP or cGMP are essential for degradative precision at different developmental stages. We discuss the diverse PDEs, the nucleotide cyclases, and the target proteins for cAMP and cGMP in Dictyostelium. We further outline the major molecular, cellular, and developmental events regulated by cyclic nucleotide signaling, with emphasis on the input of each PDE and consequence of loss-of-function mutations. Finally, we relate the structures and functions of the Dictyostelium PDEs with those of humans and in the context of potential therapeutic understandings.
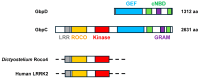
Keywords: GPCRs; LRR/ROCO kinases; PDEs; Protein kinase A; adenylyl cyclases; cAMP; cGMP; guanylyl cyclases.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures


Similar articles
-
Seven Dictyostelium discoideum phosphodiesterases degrade three pools of cAMP and cGMP.Biochem J. 2007 Feb 15;402(1):153-61. doi: 10.1042/BJ20061153. Biochem J. 2007. PMID: 17040207 Free PMC article.
-
Roles of phosphodiesterases in the regulation of the cardiac cyclic nucleotide cross-talk signaling network.J Mol Cell Cardiol. 2016 Feb;91:215-27. doi: 10.1016/j.yjmcc.2016.01.004. Epub 2016 Jan 7. J Mol Cell Cardiol. 2016. PMID: 26773602 Free PMC article.
-
Interaction between phosphodiesterases in the regulation of the cardiac β-adrenergic pathway.J Mol Cell Cardiol. 2015 Nov;88:29-38. doi: 10.1016/j.yjmcc.2015.09.011. Epub 2015 Sep 23. J Mol Cell Cardiol. 2015. PMID: 26388264 Free PMC article.
-
The perspective of cAMP/cGMP signaling and cyclic nucleotide phosphodiesterases in aortic aneurysm and dissection.Vascul Pharmacol. 2024 Mar;154:107278. doi: 10.1016/j.vph.2024.107278. Epub 2024 Jan 21. Vascul Pharmacol. 2024. PMID: 38262506 Free PMC article. Review.
-
Phosphodiesterases in the central nervous system.Handb Exp Pharmacol. 2009;(191):71-92. doi: 10.1007/978-3-540-68964-5_5. Handb Exp Pharmacol. 2009. PMID: 19089326 Review.
References
-
- van Haastert P.J.M., Keizer-Gunnink I., Pots H., Ortiz-Mateos C., Veltman D., van Egmond W., Kortholt A. Forty-five years of cGMP research in Dictyostelium: Understanding the regulation and function of the cGMP pathway for cell movement and chemotaxis. Mol. Biol. Cell. 2021;32:ar8. doi: 10.1091/mbc.E21-04-0171. - DOI - PMC - PubMed
-
- Jaiswal P., Meena N.P., Chang F.S., Liao X.H., Kim L., Kimmel A.R. An integrated, cross-regulation pathway model involving activating/adaptive and feed-forward/feed-back loops for directed oscillatory cAMP signal-relay/response during the development of Dictyostelium. Front. Cell Dev. Biol. 2023;11:1263316. doi: 10.3389/fcell.2023.1263316. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

