ALKBH5 Improves the Epithelial Cell Tight Junctions to Inhibit Escherichia coli-Induced Mastitis
- PMID: 40214476
- PMCID: PMC11988031
- DOI: 10.3390/cells14070521
ALKBH5 Improves the Epithelial Cell Tight Junctions to Inhibit Escherichia coli-Induced Mastitis
Abstract
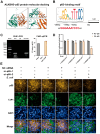
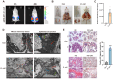
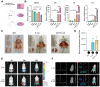
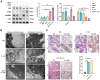
Mastitis poses a severe threat to the global cattle industry, causing huge economic losses. Environmental mastitis is mainly induced by Escherichia coli (E. coli), and the current treatment is still using antibiotics, with problems such as drug resistance and food safety. ALKBH5 is an RNA m6A demethylase that plays an important role in various biological processes, while p65 is a key regulator of inflammatory responses. Therefore, studying the interaction between ALKBH5 and p65 in protecting the mammary epithelial barrier provides new insights into the pathogenesis of mastitis. This study revealed that E. coli-induced acute inflammation activated the NF-κB/p65 signaling pathway and disrupted mammary epithelial cell tight junctions. Knockdown of ALKBH5 promoted p65 phosphorylation and inhibited the expressions of the tight junction proteins TJP1, CDH1, and OCLN. Furthermore, motif analysis, CHIP-PCR, and dual luciferase assay confirmed that phosphorylated p65 inhibited TJP1 promoter activity, thereby inhibiting TJP1 expression. In addition, the mouse experiment further demonstrated that knockdown of ALKBH5 aggravated E. coli-induced acute mastitis and epithelial cell tight junction disruption, and promoted E. coli invasion and proliferation. Significantly, this study is the first to demonstrate the details of the interaction between p65 and TJP1 and to declare the molecular mechanism of ALKBH5 in improving the cell tight junction, which lays a potential target and theoretical foundation for the treatment of mastitis and other infectious diseases.
Keywords: ALKBH5; E. coli; TJP1; mastitis; p65; tight junction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sharun K., Dhama K., Tiwari R., Gugjoo M.B., Yatoo M.I., Patel S.K., Pathak M., Karthik K., Khurana S.K., Singh R., et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021;41:107–136. doi: 10.1080/01652176.2021.1882713. - DOI - PMC - PubMed
-
- Morales-Ubaldo A.L., Rivero-Perez N., Valladares-Carranza B., Velázquez-Ordoñez V., Delgadillo-Ruiz L., Zaragoza-Bastida A. Bovine mastitis, a worldwide impact disease: Prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 2023;21:100306. doi: 10.1016/j.vas.2023.100306. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

