A New Method for Accelerated Aging of Nanoparticles to Assess the Colloidal Stability of Albumin-Coated Magnetic Nanoparticles
- PMID: 40214521
- PMCID: PMC11990806
- DOI: 10.3390/nano15070475
A New Method for Accelerated Aging of Nanoparticles to Assess the Colloidal Stability of Albumin-Coated Magnetic Nanoparticles
Abstract
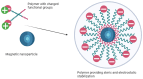
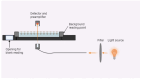
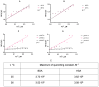
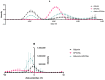
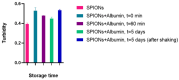
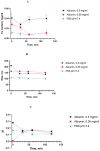
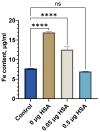
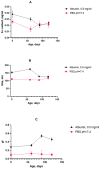
The colloidal long-storage stability of nanosized drugs is a crucial factor for pharmacology, as they require much time for robust estimation. The application of bioavailable magnetic nanosuspensions in theranostics is limited by incomplete information about their colloidal stability in the internal media of human organisms. A method for the accelerated temperature stress "aging" of magnetic nanosized suspensions is proposed for the rapid assessment and prediction of the colloidal stability over time of nanosized iron oxide suspensions stabilized by albumin HSA. Colloidal stability is assessed using dynamic light scattering (DLS), fluorescence spectroscopy, electrophoresis, and ion monitoring methods during short- and long-term storage. Rapid assessment is achieved by short high-temperature (70 °C) processing of carboxymethyl-dextran-coated nanosol in the presence of albumin. The role of albumin in the sustained stability of superparamagnetic iron oxide particles (SPIONs) was studied under conditions mimicking blood plasma (pH = 7.4) and endolysosomal cell compartments (pH = 5.5). According to the fluorescence quenching and DLS data, colloidal stability is ensured by the formation of an HSA corona on carboxymethyl-dextran-coated SPIONs and their process of clustering. In the presence of albumin, the colloidal stability of nanoparticles is shown to increase from 80 to 121 days at a storage temperature of 8 °C The prognostic shelf life of magnetic nanosol is estimated by calculating the Van't Hoff's relation for the rate of chemical reactions. The validity of using the Van't Hoff's rule is confirmed by the agreement of the calculated activation energy at 8 °C and 70 °C. The developed method of the accelerated aging of nanoparticles can not only be employed for the estimation of the shelf life of magnetic nanoparticles coated with HSA in vitro but also for assessing the stability of SPIONs applied in vivo.
Keywords: SPIONs; metal oxide nanoparticles; method of accelerated aging; nanoparticle aggregation; nanoparticle stability; protein corona; superparamagnetic iron oxide nanoparticles.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

