Graphene-Based Impregnation into Polymeric Coating for Corrosion Resistance
- PMID: 40214532
- PMCID: PMC11990139
- DOI: 10.3390/nano15070486
Graphene-Based Impregnation into Polymeric Coating for Corrosion Resistance
Abstract
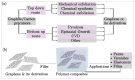
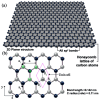
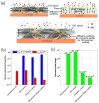
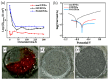
This review explores the development and application of the impregnation of graphene-based materials into polymeric coatings to enhance corrosion resistance. Derivatives of graphene, such as graphene oxide (GO) and reduced graphene oxide (rGO), have been increasingly integrated into polymer matrices to enhance polymers' mechanical, thermal, and barrier properties. Various synthesis approaches, viz., electrochemical deposition, chemical reduction, and the incorporation of functionalised graphene derivatives, have been explored for improving the dispersion and stability of graphene within polymers. These graphene-impregnated coatings have shown promising results in improving corrosion resistance by enhancing impermeability to corrosive agents and reinforcing mechanical strength under corrosive conditions. While the addition of graphene notably enhances coating performance, challenges remain in achieving uniform graphene dispersion and addressing the trade-offs between thickness and flexibility. This review highlights current advancements, limitations, and future directions, with a particular emphasis on optimising the synthesis techniques to maximise corrosion resistance while maintaining coating durability and economic feasibility.
Keywords: corrosion resistance; graphene-based nano fillers; polymer-matrix composites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Electrochemical Comparison of SAN/PANI/FLG and ZnO/GO Coated Cast Iron Subject to Corrosive Environments.Materials (Basel). 2018 Nov 11;11(11):2239. doi: 10.3390/ma11112239. Materials (Basel). 2018. PMID: 30423876 Free PMC article.
-
Biodegradation and mechanical performance of Silane-chitosan-graphene oxide composite coating on AZ31 magnesium alloys for biomedical applications.Int J Biol Macromol. 2025 Jan;287:138568. doi: 10.1016/j.ijbiomac.2024.138568. Epub 2024 Dec 7. Int J Biol Macromol. 2025. PMID: 39653235
-
Poly (3,4-ethylenedioxythiophene) graphene oxide composite coatings for controlling magnesium implant corrosion.Acta Biomater. 2017 Jan 15;48:530-540. doi: 10.1016/j.actbio.2016.11.039. Epub 2016 Nov 17. Acta Biomater. 2017. PMID: 27867108 Free PMC article.
-
Recent advancements in mechanical properties of graphene-enhanced polymer nanocomposites: Progress, challenges, and pathways forward.J Mol Graph Model. 2025 Mar;135:108908. doi: 10.1016/j.jmgm.2024.108908. Epub 2024 Nov 15. J Mol Graph Model. 2025. PMID: 39579712 Review.
-
A Review of the Impact of Graphene Oxide on Cement Composites.Nanomaterials (Basel). 2025 Jan 29;15(3):216. doi: 10.3390/nano15030216. Nanomaterials (Basel). 2025. PMID: 39940192 Free PMC article. Review.
References
-
- Koch G. 1—Cost of corrosion. In: El-Sherik A.M., editor. Trends in Oil and Gas Corrosion Research and Technologies. Woodhead Publishing; Boston, MA, USA: 2017. pp. 3–30. Woodhead Publishing Series in Energy.
-
- Kumar Gupta R., Malviya M., Verma C.K., Gupta N.A., Quraishi M. Pyridine-based functionalized graphene oxides as a new class of corrosion inhibitors for mild steel: An experimental and DFT approach. RSC Adv. 2017;7:39063–39074.
-
- Boutoumit A., Elhawary M., Bellaouchou A., Boudalia M., Hammani O., José Garcia A., Amin H.M.A. Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl. Coatings. 2024;14:783. doi: 10.3390/coatings14070783. - DOI
-
- Najem A., Campos O.S., Girst G., Raji M., Hunyadi A., García-Antón J., Bellaouchou A., Amin H.M.A., Boudalia M. Experimental and DFT Atomistic Insights into the Mechanism of Corrosion Protection of Low-Carbon Steel in an Acidic Medium by Polymethoxyflavones from Citrus Peel Waste. J. Electrochem. Soc. 2023;170:093512
Publication types
LinkOut - more resources
Full Text Sources

