First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces
- PMID: 40214547
- PMCID: PMC11990154
- DOI: 10.3390/nano15070502
First-Principles Calculations for Glycine Adsorption Dynamics and Surface-Enhanced Raman Spectroscopy on Diamond Surfaces
Abstract
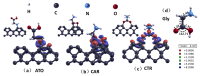
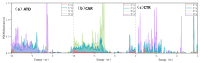
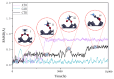
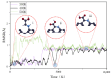
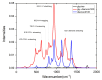
Based on first-principles calculations, the stability of three adsorption configurations of glycine on the (100) surface of diamonds was studied, leading to an investigation into the surface-enhanced Raman scattering (SERS) effect of the diamond substrate. The results showed that the carboxyl-terminated adsorption configuration (CAR) was the most stable and shortest interface distance compared to other configurations. This stability was primarily attributed to the formation of strong polar covalent bonds between the carboxyl O atoms and the surface C atoms of the (100) surface of diamonds. These results were further corroborated by first-principles molecular dynamics simulations. Within the temperature range of 300 to 500 K, the glycine molecules in the carboxyl-terminated adjacent-dimer phenyl-like (CAR) configuration exhibited only simple thermal vibrations with varying amplitudes. In contrast, the metastable ATO and carboxyl-terminated trans-dimer phenyl-like ring (CTR) configurations were observed to gradually transform into benzene-ring-like structures akin to the CAR configuration. After adsorption, the intensity of glycine's characteristic peaks increased substantially, accompanied by a blue shift phenomenon. Notably, the characteristic peaks related to the carboxyl and amino groups exhibited the highest enhancement amplitude, exceeding 200 times, with an average enhancement amplitude exceeding 50 times. The diamond substrate, with its excellent adsorption properties and strong surface Raman spectroscopy characteristics, represents a highly promising candidate in the field of biomedicine.
Keywords: AIMD; SERS; diamond surface; first-principles calculation; glycine.
Conflict of interest statement
Author J.Y. was employed by the company Beiben Trucks Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Investigation of the Effect of Molecules Containing Sulfonamide Moiety Adsorbed on the FAPbI3 Perovskite Surface: A First-Principles Study.Molecules. 2025 Jun 4;30(11):2463. doi: 10.3390/molecules30112463. Molecules. 2025. PMID: 40509350 Free PMC article.
-
First-principles investigation of oxidized Si- and Ge-terminated diamond (100) surfaces.J Chem Phys. 2024 Aug 14;161(6):064702. doi: 10.1063/5.0203185. J Chem Phys. 2024. PMID: 39120035
-
Structure of monolayers formed from neurotensin and its single-site mutants: vibrational spectroscopic studies.J Phys Chem B. 2011 May 26;115(20):6709-21. doi: 10.1021/jp200805f. Epub 2011 May 4. J Phys Chem B. 2011. PMID: 21542591
-
Lighting up the Raman signal of molecules in the vicinity of graphene related materials.Acc Chem Res. 2015 Jul 21;48(7):1862-70. doi: 10.1021/ar500466u. Epub 2015 Jun 9. Acc Chem Res. 2015. PMID: 26056861 Review.
-
Application of surface-enhanced Raman scattering to qualitative and quantitative analysis of arsenic species.Anal Methods. 2023 Sep 28;15(37):4798-4810. doi: 10.1039/d3ay00736g. Anal Methods. 2023. PMID: 37724459 Review.
References
-
- Carré A., Lacarrière V. How substrate properties control cell adhesion. A physical–chemical approach. J. Adhes. Sci. Technol. 2010;24:815–830. doi: 10.1163/016942409X12598231567862. - DOI
-
- Satyanarayana U. Biochemistry. Elsevier Health Sciences; Berlin/Heidelberg, Germany: 2013. pp. 43–69.
-
- Lopes I., Piao L., Stievano L., Lambert J.-F. Adsorption of amino acids on oxide supports: A solid-state NMR study of glycine adsorption on silica and alumina. J. Phys. Chem. C. 2009;113:18163–18172. doi: 10.1021/jp906891y. - DOI
-
- Lei G., Xun S., Wei S. Adsorption of Amino Acid Inhibitors on Iron Surface: A First-principles Investigation. Surf. Technol. 2017;46:228–234.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

