Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms
- PMID: 40214555
- PMCID: PMC11990413
- DOI: 10.3390/nano15070510
Carbon-Infiltrated Carbon Nanotube Topography Reduces the Growth of Staphylococcus aureus Biofilms
Abstract
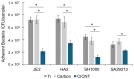
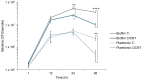
Orthopedic implant-associated infections are a growing problem. These infections are often associated with bacterial biofilms, such as those formed by Staphylococcus aureus. Nanotextured surfaces can reduce or prevent the development of bacterial biofilms and could help reduce infection rates and severity. Previous work has shown that a carbon-infiltrated carbon nanotube (CICNT) surface reduces the growth of S. aureus biofilms. This work expands on previous experiments, showing that the topography of the CICNT, rather than its surface chemistry, is responsible for the reduction in biofilm growth. Additionally, the CICNT surface does not reduce biofilm growth by killing the bacteria or by preventing their attachment. Rather it likely slows cell growth, resulting in fewer cells and reduced biofilm formation.
Keywords: MRSA; Staphylococcus aureus; biofilm; carbon nanotubes; nanostructured surface.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

