Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System
- PMID: 40214601
- PMCID: PMC11990697
- DOI: 10.3390/nano15070558
Factors Influencing Removal of Trichloroethylene in a Zero-Valent Iron Fenton System
Abstract
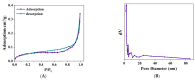
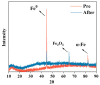
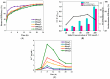
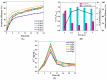
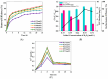
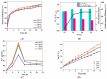
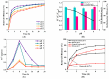
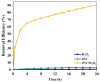
Trichloroethylene (TCE), a volatile organic compound commonly used as a solvent, is frequently detected in contaminated groundwater. In the zero-valent iron (ZVI) Fenton process, TCE can be eventually dechlorinated into non-toxic products, which is mainly caused by hydroxyl radicals derived from H2O2. However, some key factors in the dechlorination of TCE in the zero-valent iron Fenton process have not been studied clearly. In the present study, the effects of the initial TCE concentration, initial H2O2 concentration, dosage of ZVI, initial pH, and temperature on TCE degradation in the ZVI Fenton process were studied. In addition, the structure and surface morphology of the ZVI used in this study were analyzed through scanning electron microscopy (SEM), N2 adsorption-desorption, and X-ray diffractometry (XRD). The experimental results demonstrated that the dosage of ZVI and initial H2O2 concentration had obvious impacts on TCE degradation. At a ZVI dosage of 2 g/L and an initial H2O2 concentration of 0.53 mol/L, more than 97% of TCE could be degraded within 24 h at 25 °C. We found that the ZVI Fenton process could efficiently degrade TCE at a broad pH range and room temperature, making it applicable to groundwater remediation. TCE degradation was associated with Fe2+ concentration. Spectroscopic analyses indicated that the oxide film formed on the ZVI surface was associated with Fe2+ concentration in enhanced TCE dechlorination. The ZVI Fenton process could work at a wide range of TCE concentrations (0-200 mg/L).
Keywords: Fenton process; groundwater; trichloroethylene (TCE); zero-valent iron (ZVI).
Conflict of interest statement
Authors declare no conflict of interest.
Figures

References
-
- Doherty R.E. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 2—Trichloroethylene and 1,1,1-Trichloroethane. Environ. Forensics. 2000;1:83–93. doi: 10.1006/enfo.2000.0011. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

