Tripodal Silanolate Ligands Expand [MoX3] Chemistry Beyond Its Traditional Borders
- PMID: 40214616
- PMCID: PMC12022994
- DOI: 10.1021/jacs.5c02178
Tripodal Silanolate Ligands Expand [MoX3] Chemistry Beyond Its Traditional Borders
Abstract
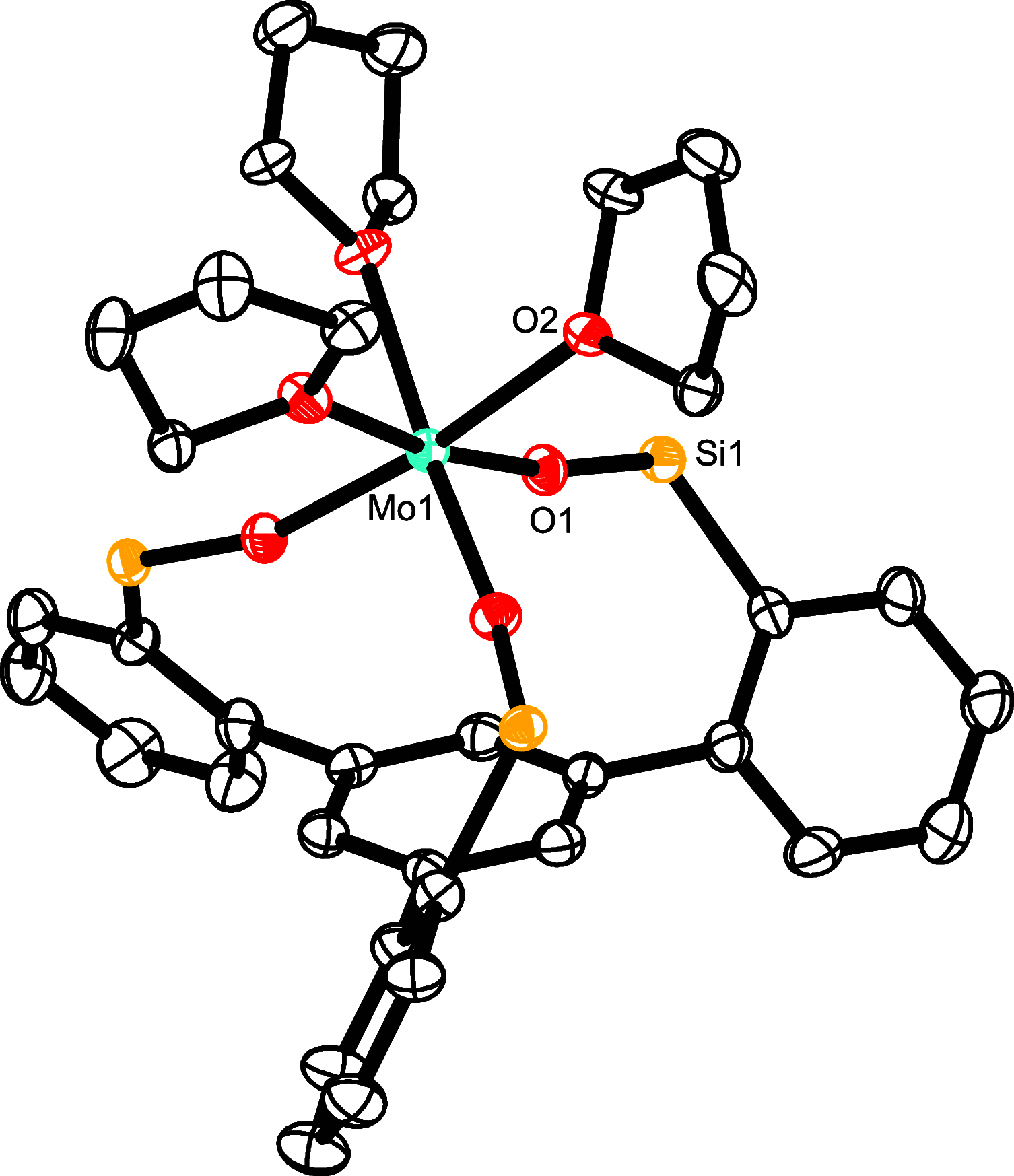
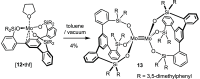
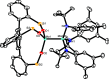
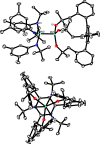
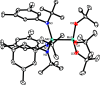
Homodimeric complexes [X3Mo≡MoX3] are commonplace, but in no case is the corresponding monomeric [MoX3] species known; conversely, none of the very rare monomeric complexes [MoX3] has the respective homodimeric analogue. This mutual exclusivity ends with the present study; on top, an entirely unprecedented class of heterodimers of type [X3Mo≡MoY3] is reported. Key to success was the use of tripodal silanolates as ancillary ligands; the fence formed by properly chosen peripheral substituents shields the sensitive Mo(+3) center; homodimerization of the resulting [MoX3] complexes is then kinetically strongly disfavored, though possible. The monomers are able to cleave N2O and convert gem-dihalides into metal alkylidynes; they exist in different binding modes, in which the basal phenyl ring of the ligand backbone is either completely unengaged with the central metal or tightly bound to it, depending on whether the ligand sphere is complemented by solvent molecules or not. If the latter are sufficiently labile, a surprisingly facile heterodimerization of the d3 electron fragments will ensue; the resulting products [X3Mo≡MoY3] incorporate the intact Cummins complex [(tBu)(Ar)N]3Mo (Ar = 3,5-dimethylphenyl) as one of their constituents, which is famous for not engaging in metal-metal triple bonding otherwise. Heterodimerization was also observed with simple tert-butoxide ligands. The new type of heterodimers features unusually long yet robust Mo≡Mo bonds, which are notably polarized according to DFT. However, there is no direct correlation between the extreme Mo≡Mo bond lengths and the strikingly deshielded 95Mo NMR signals, since ligand-based orbitals can also markedly affect the shielding tensor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





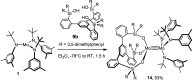

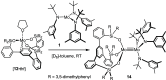


Similar articles
-
Molybdenum(VI) Nitrido Complexes with Tripodal Silanolate Ligands. Structure and Electronic Character of an Unsymmetrical Dimolybdenum μ-Nitrido Complex Formed by Incomplete Nitrogen Atom Transfer.Inorg Chem. 2024 May 6;63(18):8376-8389. doi: 10.1021/acs.inorgchem.4c00762. Epub 2024 Apr 25. Inorg Chem. 2024. PMID: 38663089 Free PMC article.
-
Synthesis of molybdenum nitrido complexes for triple-bond metathesis of alkynes and nitriles.Inorg Chem. 2011 Jul 4;50(13):5936-45. doi: 10.1021/ic1024247. Epub 2011 Jun 1. Inorg Chem. 2011. PMID: 21630685
-
Canopy Catalysts for Alkyne Metathesis: Investigations into a Bimolecular Decomposition Pathway and the Stability of the Podand Cap.Chemistry. 2021 Oct 7;27(56):14025-14033. doi: 10.1002/chem.202102080. Epub 2021 Aug 26. Chemistry. 2021. PMID: 34293239 Free PMC article.
-
Organometallic Chemistry of Transition Metal Alkylidyne Complexes Centered at Metathesis Reactions.J Am Chem Soc. 2022 Jul 20;144(28):12546-12566. doi: 10.1021/jacs.2c01192. Epub 2022 Jul 6. J Am Chem Soc. 2022. PMID: 35793547 Review.
-
Expanding the Rare-Earth Metal BINOLate Catalytic Multitool beyond Enantioselective Organic Synthesis.Acc Chem Res. 2021 Jun 1;54(11):2637-2648. doi: 10.1021/acs.accounts.1c00148. Epub 2021 May 20. Acc Chem Res. 2021. PMID: 34014657 Review.
Cited by
-
Synergistic C-H bond activation across molybdenum-iridium multiply bonded complexes: a cascade of transformations.Chem Sci. 2025 Jul 7;16(32):14564-14577. doi: 10.1039/d5sc03465e. eCollection 2025 Aug 13. Chem Sci. 2025. PMID: 40698160 Free PMC article.
References
-
- Huq F.; Mowat W.; Shortland A.; Skapski A. C.; Wilkinson G. Crystal structure of hexakis(trimethylsilylmethyl)-dimolybdenum. J. Chem. Soc. D: Chem. Commun. 1971, 1079–1080. 10.1039/c29710001079. - DOI
- Yagupsky G.; Mowat W.; Shortland A.; Wilkinson G. Trimethylsilylmethyl compounds of transition metals. J. Chem. Soc. D: Chem. Commun. 1970, 1369–1370. 10.1039/c29700001369. - DOI
-
The compound had originally been misassigned as a monomeric Mo(+4) tetraalkyl species, see
-
- Cotton F. A.; Murillo C. A.; Walton R. A.. Multiple Bonds Between Metal Atoms., 3rd ed.; Springer: New York, 2005.
-
- Chisholm M. H. The coordination chemistry of dinuclear molybdenum(III) and tungsten(III): d3-d3 dimers. Acc. Chem. Res. 1990, 23 (12), 419–425. 10.1021/ar00180a004. - DOI
-
- Chetcuti M. J.; Chisholm M. H.; Folting K.; Haitko D. A.; Huffman J. C.; Janos J. 1,2-Dibenzyl- and 1,2-diaryltetrakis-(dimethylamido)dimolybdenum and -ditungsten compounds: M2R2(NMe2)4 (M≡M). Structural effects of Me2N-to-M π-bonding. J. Am. Chem. Soc. 1983, 105 (5), 1163–1170. 10.1021/ja00343a015. - DOI
-
- Krackl S.; Ma J.-G.; Aksu Y.; Driess M. Facile Access to Homo- and Heteroleptic, Triply Bonded Dimolybdenum Hexaalkoxides with Unsaturated Alkoxide Ligands. Eur. J. Inorg. Chem. 2011, 2011, 1725–1732. 10.1002/ejic.201001236. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

