Levodopa treatment: impacts and mechanisms throughout Parkinson's disease progression
- PMID: 40214767
- PMCID: PMC12116664
- DOI: 10.1007/s00702-025-02893-4
Levodopa treatment: impacts and mechanisms throughout Parkinson's disease progression
Abstract
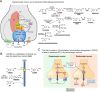
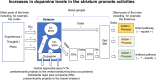
Treatment with levodopa, a precursor of dopamine (DA), to compensate for the loss of endogenous DA in Parkinson's disease (PD), has been a success story for over 50 years. However, in late stages of PD, the progressive degeneration of dopaminergic neurons and the ongoing reduction in endogenous DA concentrations make it increasingly difficult to maintain normal-like DA function. Typically, in late PD, higher doses of levodopa are required, and the fluctuations in striatal DA concentrations-reflecting the timing pattern of levodopa administrations-become more pronounced. These DA fluctuations can include highs that induce involuntary movements (levodopa-induced dyskinesia, LID) or lows that result in insufficient suppression of PD symptoms ("OFF" phases). The enhanced fluctuations primarily arise from the loss of DA buffering capacity, resulting from the degeneration of DA neurons, and an increased reliance on levodopa-derived DA release as a "false neurotransmitter" by serotonergic neurons. In many patients, the LID and OFF-phases can be alleviated by modifying the levodopa therapy to provide a more continuous delivery or by using additional medications, such as monoamine oxidase-B (MAO-B) inhibitors, amantadine, or dopaminergic receptor agonists. Understanding the challenges faced by levodopa therapy also requires considering that the PD striatum is characterized not only by the loss of DA neurons but also by neuroplastic adaptations and PD-induced degenerations of other neural populations. This review provides a broad overview on the use of levodopa in treating PD, with a focus on the underlying science of the challenges encountered in late stages of the disease.
Keywords: Levodopa; Levodopa-induced dyskinesia (LID); Long duration response (LDR); Mode of action; OFF-phase; Parkinson’s disease; Progression.
© 2025. The Author(s).
Figures


References
-
- Amjad F, Bhatti D, Davis TL, Oguh O, Pahwa R, Kukreja P, Zamudio J, Metman LV (2019) Current practices for outpatient initiation of levodopa-carbidopa intestinal gel for management of advanced Parkinson’s disease in the United States. Adv Ther 36:2233–2246. 10.1007/s12325-019-01029-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

