Radioactivity levels in the saliva of patients undergoing targeted radioligand therapy with [177Lu]Lu-PSMA-I&T and [177Lu]Lu-DOTA-TOC
- PMID: 40214824
- PMCID: PMC11991938
- DOI: 10.1007/s00784-025-06300-w
Radioactivity levels in the saliva of patients undergoing targeted radioligand therapy with [177Lu]Lu-PSMA-I&T and [177Lu]Lu-DOTA-TOC
Abstract
Objectives: The number of patients receiving radioligand therapy (RLT) has risen sharply in recent years. This raises concerns about possible risks to dental healthcare workers due to their exposure to the patients and their saliva. We therefore set about to measure the salivary radioactivity in patients undergoing 177Lu-RLT.
Materials and methods: We recruited in-house RLT patients receiving [177Lu]Lu -DOTA-TOC (n = 6) or [177Lu]Lu-PSMA-I&T (n = 14). We measured the radioactivity concentrations in 1 ml saliva samples collected before and 0.5, 2, 4, 21, 27, and 45 h post application of the radioligands, with additional samples collected at 51 and 69 h for [177Lu]Lu-PSMA-I&T patients. The biological half-life (BHL) and area under the curve (AUC) were calculated for the radioactivity of the saliva for both cohorts.
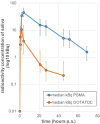
Results: Both cohorts exhibited increases in salivary radioactivity, attaining peaks at 2 h p.i. of [177Lu]Lu-DOTA-TOC and 4 h p.i. of [177Lu]Lu-PSMA-I&T, and presenting with a significant decrease until the patients discharge. The median peak concentration for [177Lu]Lu-PSMA-I&T was four-fold higher than for the [177Lu]Lu-DOTA-TOC group. For PSMA-patients, the BHL was 14 h and the mean AUC was 895 kBqh/ml. For DOTA-TOC patients, these values were 8.5 h and 96 kBqh/ml, respectively.
Conclusion: Salivary radioactivity peaks earlier and at lower levels in [177Lu]Lu-DOTA-TOC patients compared to [177Lu]Lu-PSMA-I&T, which shows longer retention and ten times higher radioactivity turnover in saliva. However, radiation exposure to medical staff by the patents saliva can be considered minimal.
Clinical relevance: Salivary radioactivity of patients undergoing 177Lu-RLT poses minimal risk to oral healthcare workers.
Keywords: 177Lu-PSMA; MRONJ; PSMA; Prostate cancer; Radionuclide therapy; Saliva.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Disclosure: No potential conflict of interest relevant to this article was reported. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Baseline clinical characteristics predict overall survival in patients undergoing radioligand therapy with [177Lu]Lu-PSMA I&T during long-term follow-up.Eur J Nucl Med Mol Imaging. 2022 Oct;49(12):4262-4270. doi: 10.1007/s00259-022-05853-2. Epub 2022 Jun 2. Eur J Nucl Med Mol Imaging. 2022. PMID: 35650263 Free PMC article.
-
Individual radiosensitivity reflected by γ-H2AX and 53BP1 foci predicts outcome in PSMA-targeted radioligand therapy.Eur J Nucl Med Mol Imaging. 2023 Jan;50(2):602-612. doi: 10.1007/s00259-022-05974-8. Epub 2022 Sep 22. Eur J Nucl Med Mol Imaging. 2023. PMID: 36136101 Free PMC article.
-
Personalized dosimetry assessment of [177Lu]Lu-PSMA-617 radioligand therapy in the management of metastatic castration-resistant prostate cancer.Int J Radiat Biol. 2024;100(11):1551-1559. doi: 10.1080/09553002.2024.2404448. Epub 2024 Sep 20. Int J Radiat Biol. 2024. PMID: 39302840
-
[PSMA radioligand therapy in patients with advanced prostate cancer].Urologe A. 2020 Jun;59(6):680-686. doi: 10.1007/s00120-020-01205-w. Urologe A. 2020. PMID: 32333064 Review. German.
-
[177Lu]Lu-PSMA-Radioligand Therapy Efficacy Outcomes in Taxane-Naïve Versus Taxane-Treated Patients with Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Metaanalysis.J Nucl Med. 2023 Aug;64(8):1266-1271. doi: 10.2967/jnumed.123.265414. Epub 2023 May 11. J Nucl Med. 2023. PMID: 37169534
References
-
- Fizazi K et al (2023) Health-related quality of life and pain outcomes with [(177)Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 24(6):597–610 - PMC - PubMed
-
- Parghane RV, Basu S (2023) PSMA-targeted radioligand therapy in prostate cancer: current status and future prospects. Expert Rev Anticancer Ther 23(9):959–975 - PubMed
-
- Kwekkeboom DJ et al (2010) Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 17(1):R53–73 - PubMed
-
- Khan S et al (2011) Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med 52(9):1361–1368 - PubMed
-
- Bergsma H et al (2012) Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Pract Res Clin Gastroenterol 26(6):867–881 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

