Beyond the blood-brain barrier: feasibility and technical validation of dual-compartment circulating tumor cells detection in high-grade glioma patients
- PMID: 40214852
- PMCID: PMC11991960
- DOI: 10.1007/s10143-025-03511-3
Beyond the blood-brain barrier: feasibility and technical validation of dual-compartment circulating tumor cells detection in high-grade glioma patients
Abstract
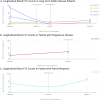
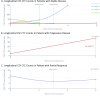
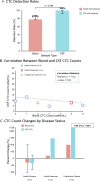
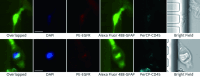
The elusive nature of brain tumor progression, hidden behind the blood-brain barrier, presents significant challenges for treatment monitoring in high-grade gliomas. In this feasibility study, we evaluate a novel approach to tracking glioblastoma through liquid biopsy, assessing whether tumor cells leave detectable molecular footprints in both blood and cerebrospinal fluid (CSF). Using the MiSelect R II System with specialized microfluidic technology, we analyzed paired blood and CSF samples from six glioblastoma patients, revealing a striking presence of circulating tumor cells (CTCs)- with higher abundance in CSF, where detection rates reached 100% compared to 83.3% in blood. Our technical validation demonstrates the system's capability to identify CTCs through multi-marker analysis (EGFR+/GFAP+/CD45-). Preliminary observations revealed higher CTC counts in CSF (median 15.5 cells/mL) compared to blood (median 3.0 cells/mL), with notable differences between compartments suggesting they may reflect distinct aspects of disease biology. In a patient who developed progressive disease, we observed a substantial increase in CSF CTCs from 14 to 116 cells/mL, warranting further investigation in larger cohorts. Additionally, we detected CTC clusters in both compartments, an intriguing finding with potential biological significance. While our interim analysis provides technical proof-of-concept for CTC detection in glioblastoma patients, the limited sample size precludes definitive conclusions regarding clinical utility. These findings establish a methodological foundation for future comprehensive studies exploring the relationship between CTC dynamics and clinical outcomes in high-grade gliomas.
Keywords: Biomarkers; Cerebrospinal fluid; Circulating tumor cells (CTCs); Glioblastoma; High-grade glioma; Liquid biopsy; MiSelect R II system; Microfluidic technology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Review Board of China Medical University and Hospital in Taiwan (approval No. CMUH112-REC2-103). The trial is registered at ClinicalTrials.gov (registration number pending). The research in accordance with local regulations and international standards, including the Declaration of Helsinki. Consent to participate: Informed consent was obtained from all individual participants included in the study. Competing interests: The authors declare no competing interests.
Figures
References
-
- Fabro F, Lamfers MLM, Leenstra S (2022) Advancements, challenges, and future directions in tackling glioblastoma resistance to small kinase inhibitors. Cancers (Basel), 14(3)
-
- Fisher JP, Adamson DC (2021) Current FDA-Approved therapies for High-Grade malignant gliomas. Biomedicines, 9(3)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

