AhR Activation at the Air-Blood Barrier Alters Systemic microRNA Release After Inhalation of Particulate Matter Containing Environmentally Persistent Free Radicals
- PMID: 40214911
- PMCID: PMC12018632
- DOI: 10.1007/s12012-025-09989-z
AhR Activation at the Air-Blood Barrier Alters Systemic microRNA Release After Inhalation of Particulate Matter Containing Environmentally Persistent Free Radicals
Abstract
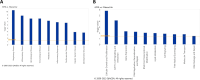
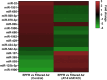
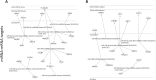
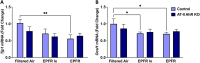
Particulate matter containing environmentally persistent free radicals (EPFRs) is formed when organic pollutants are incompletely burned and adsorb to the surface of particles containing redox-active metals. Our prior studies showed that in mice, EPFR inhalation impaired vascular relaxation in a dose- and endothelium-dependent manner. We also observed that activation of the aryl hydrocarbon receptor (AhR) in the alveolar type-II (AT-II) cells that form the air-blood interface stimulates the release of systemic factors that promote endothelial dysfunction in vessels peripheral to the lung. AhR is a recognized regulator of microRNA (miRNA) biogenesis, and miRNA control diverse signaling pathways. We thus hypothesized that systemic EPFR-induced vascular endothelial dysfunction is initiated via AhR activation in AT-II cells, resulting in a systemic release of miRNA. Using a combustion reactor, we generated EPFR of two free radical concentrations-EPFRlo (1016-17 radicals/g particles) and EPFR (1018-19 radicals/g)-and exposed mice by inhalation. EFPR inhalation resulted in changes in a distinct array of miRNA in the plasma, and these miRNAs are linked to multiple systemic effects, including cardiovascular diseases and dysregulation of cellular and molecular pathways associated with cardiovascular dysfunction. We identified 17 miRNA in plasma that were altered dependent upon both AhR activation in AT-II cells and ~ 280 ug/m3 EPFR exposure. Using Ingenuity Pathway Analysis, we found that 5 of these miRNAs have roles in modulating endothelin-1 and endothelial nitric oxide signaling, known regulators of endothelial function. Furthermore, EPFR exposure reduced the expression of lung adherens and gap junction proteins in control mice but not AT-II-AhR deficient mice, and reductions in barrier function may facilitate miRNA release from the lungs. In summary, our findings support that miRNA may be systemic mediators promoting endothelial dysfunction mediated via EPFR-induced AhR activation at the air-blood interface.
Keywords: Air pollution; Alveolar type-II cells; Aryl hydrocarbon receptor; Cardiovascular health; Environmental toxicology; Environmentally persistent free radicals; Inhalation; Particulate matter; microRNA.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures

Similar articles
-
Inhalation of particulate matter containing environmentally persistent free radicals induces endothelial dysfunction mediated via AhR activation at the air-blood interface.Toxicol Sci. 2024 May 28;199(2):246-260. doi: 10.1093/toxsci/kfae007. Toxicol Sci. 2024. PMID: 38310335 Free PMC article.
-
Inhalation of particulate matter containing free radicals leads to decreased vascular responsiveness associated with an altered pulmonary function.Am J Physiol Heart Circ Physiol. 2021 Oct 1;321(4):H667-H683. doi: 10.1152/ajpheart.00725.2020. Epub 2021 Aug 20. Am J Physiol Heart Circ Physiol. 2021. PMID: 34415187 Free PMC article.
-
Particulate matter containing environmentally persistent free radicals induces AhR-dependent cytokine and reactive oxygen species production in human bronchial epithelial cells.PLoS One. 2018 Oct 11;13(10):e0205412. doi: 10.1371/journal.pone.0205412. eCollection 2018. PLoS One. 2018. PMID: 30308017 Free PMC article.
-
Addressing Emerging Risks: Scientific and Regulatory Challenges Associated with Environmentally Persistent Free Radicals.Int J Environ Res Public Health. 2016 Jun 8;13(6):573. doi: 10.3390/ijerph13060573. Int J Environ Res Public Health. 2016. PMID: 27338429 Free PMC article. Review.
-
Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways.Toxicol Lett. 2017 Mar 15;270:88-95. doi: 10.1016/j.toxlet.2017.01.017. Epub 2017 Feb 9. Toxicol Lett. 2017. PMID: 28189649 Review.
References
-
- Kumar, P., Singh, A., Arora, T., Singh, S., & Singh, R. (2023). Critical review on emerging health effects associated with the indoor air quality and its sustainable management. Science of the Total Environment,872, 162163. - PubMed
-
- Kyu, H. H., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., Abbastabar, H., Abd-Allah, F., Abdela, J., & Abdelalim, A. (2018). Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet,392(10159), 1859–1922. - PMC - PubMed
-
- Ravindra, K., Kaur-Sidhu, M., spsampsps Mor, S. (2020). Air pollution in rural households due to solid biomass fuel use and its health impacts. Indoor environmental quality: Select proceedings of the 1st ACIEQ
-
- Langrish, J., Bosson, J., Unosson, J., Muala, A., Newby, D., Mills, N., Blomberg, A., & Sandström, T. (2012). Cardiovascular effects of particulate air pollution exposure: Time course and underlying mechanisms. Journal of Internal Medicine,272(3), 224–239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

