Rare pancreatic ductal adenocarcinoma variants and other malignant epithelial tumors: a comprehensive clinical and radiologic review
- PMID: 40214914
- PMCID: PMC12287246
- DOI: 10.1007/s11604-025-01777-7
Rare pancreatic ductal adenocarcinoma variants and other malignant epithelial tumors: a comprehensive clinical and radiologic review
Abstract
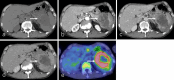
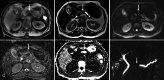
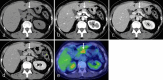
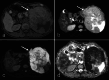
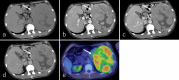
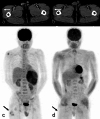
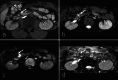
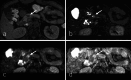
Over 95% of pancreatic carcinomas are classified as conventional pancreatic ductal adenocarcinoma (cPDAC), while less than 5% consist of rare histological subtypes. Some of these rare histological subtypes, such as colloid carcinoma, medullary carcinoma, and undifferentiated carcinoma with osteoclast-like giant cells, are associated with a relatively better prognosis compared to cPDAC, whereas others, including signet ring cell carcinoma/poorly cohesive carcinoma, adenosquamous carcinoma, large cell carcinoma with rhabdoid phenotype, and undifferentiated carcinoma, have a worse prognosis. Other malignant pancreatic epithelial tumors (MPET) include acinar cell carcinoma, pancreatoblastoma, and solid-pseudopapillary neoplasm that should also be differentiate from PDACs. Accurate differentiation among PDAC subtypes and other MPETs is essential for precise survival predictions and effective therapeutic planning. However, cPDAC, rare histological subtypes of PDAC and MPETs often exhibit similar imaging findings, making it challenging to establish a diagnosis based solely on imaging. Thus, needle biopsy or surgical resection is generally required for the final diagnosis. We herein present a review article based on case studies and literature reviews of rare histological subtypes of PDAC and other MPET, with particular focus on their imaging characteristics, referencing the 5th edition of the World Health Organization classification.
Keywords: 1⁸F-FDG-PET; CT; MRI; Pancreatic ductal adenocarcinoma; Radiological features; Rare histologic subtype.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflict of interest associated with this manuscript. Ethical approval: Ethical approval for this manuscript was waived by the institutional review board of our institution as it is a review article based on published literature and case presentations.
Figures
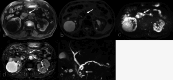
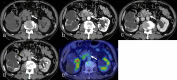
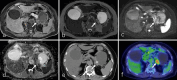
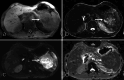
Similar articles
-
Potential Efficacy of 68 Ga-Trivehexin PET/CT and Immunohistochemical Validation of αvβ6 Integrin Expression in Patients With Head and Neck Squamous Cell Carcinoma and Pancreatic Ductal Adenocarcinoma.Clin Nucl Med. 2024 Aug 1;49(8):733-740. doi: 10.1097/RLU.0000000000005278. Epub 2024 May 14. Clin Nucl Med. 2024. PMID: 38768077
-
Multiparametric MRI for Assessment of the Biological Invasiveness and Prognosis of Pancreatic Ductal Adenocarcinoma in the Era of Artificial Intelligence.J Magn Reson Imaging. 2025 Jul;62(1):9-19. doi: 10.1002/jmri.29708. Epub 2025 Jan 9. J Magn Reson Imaging. 2025. PMID: 39781607 Review.
-
PLAGL2 as a prognostic biomarker and an EMT-promoting factor in PDAC.Sci Rep. 2025 Jul 14;15(1):25425. doi: 10.1038/s41598-025-09591-x. Sci Rep. 2025. PMID: 40659704 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma.J Thorac Oncol. 2011 Feb;6(2):244-85. doi: 10.1097/JTO.0b013e318206a221. J Thorac Oncol. 2011. PMID: 21252716 Free PMC article.
References
-
- Vital Statistics Japan reported by the Ministry of Health, Labour and Welfare (2022) [Internet]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai22/index.html.
-
- WHO Classification of Tumours Editorial Board: WHO Classification of Tumors, 5th ed, vol. 1 (2019) Digestive system tumours. Chapter 10. Tumours of the pancreas. IARC Press Lyon.
-
- Japanese Radiological Society ed. Diagnostic imaging guidelines 2021: English version. 2021. Available from: https://www.radiology.jp/content/files/gl2021/diagnostic_imaging_guideli...
-
- Schawkat K, Manning MA, Glickman JN, Mortele KJ. Pancreatic ductal adenocarcinoma and its variants: pearls and perils. Radiographics. 2020;40(5):1219–39. 10.1148/rg.2020190184. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

