Sleep disorders increase the risk of dementia, Alzheimer's disease, and cognitive decline: a meta-analysis
- PMID: 40214959
- PMCID: PMC12181552
- DOI: 10.1007/s11357-025-01637-2
Sleep disorders increase the risk of dementia, Alzheimer's disease, and cognitive decline: a meta-analysis
Abstract
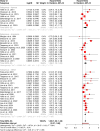
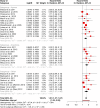
Sleep disorders, particularly insomnia and obstructive sleep apnea, are increasingly implicated as significant contributors to cognitive decline, dementia, and neurodegenerative diseases such as Alzheimer's disease (AD) and vascular cognitive impairment and dementia (VCID). However, the extent and specificity of these associations remain uncertain. This meta-analysis evaluates the impact of common sleep disorders on the risk of developing dementia and cognitive decline. A comprehensive search of the literature was conducted to identify prospective cohort studies assessing sleep disorders and dementia risk. Studies reporting risk estimates for dementia, AD, or cognitive decline associated with obstructive sleep apnea, insomnia, and other sleep disorders (e.g., restless legs syndrome, circadian rhythm sleep disorders, excessive daytime sleepiness) were included. Meta-analyses were performed using a random-effects model to calculate pooled hazard ratios (HRs) and 95% confidence intervals (CIs). Thirty-nine cohort studies were included, with subgroup analyses showing significant associations between all-cause dementia and obstructive sleep apnea (HR 1.33, 95% CI 1.09-1.61), insomnia (HR 1.36, 95% CI 1.19-1.55), and other sleep disorders (HR 1.33, 95% CI 1.24-1.43). Obstructive sleep apnea increased the risk for AD (HR 1.45, 95% CI 1.24-1.69), though its association with vascular dementia did not reach statistical significance (HR 1.35, 95% CI 0.99-1.84). Insomnia was significantly associated with increased risk for both vascular dementia (HR 1.59, 95% CI 1.01-2.51) and AD (HR 1.49, 95% CI 1.27-1.74). This meta-analysis highlights the critical role of sleep disorders in dementia risk, emphasizing the need for early detection and management of sleep disturbances. Targeted interventions could play a pivotal role in reducing dementia risk, particularly among high-risk populations.
Keywords: Aging; Apnoe; Circadian rhythms; Cognitive decline; Inadequate sleep; Neurodegeneration; Semmelweis Study; Sleep deficit; Sleep-disordered breathing; Stroke.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: NA. Consent for publication: NA. Competing interests: Dr. Balázs Győrffy serves as Associate Editor for GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and has personal relationships with individuals involved in the submission of this paper. Disclaimer: The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The 4o version of ChatGPT, developed by OpenAI, and Claude 3.5 Sonnet, developed by Anthropic were used as a language tool to refine our writing and enhance the clarity of our work.
Figures




Similar articles
-
Pharmacotherapies for sleep disturbances in dementia.Cochrane Database Syst Rev. 2016 Nov 16;11(11):CD009178. doi: 10.1002/14651858.CD009178.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Nov 15;11:CD009178. doi: 10.1002/14651858.CD009178.pub4. PMID: 27851868 Free PMC article. Updated.
-
Association between sleep apnoea and risk of cognitive impairment and Alzheimer's disease: a meta-analysis of cohort-based studies.Sleep Breath. 2024 May;28(2):585-595. doi: 10.1007/s11325-023-02934-w. Epub 2023 Oct 20. Sleep Breath. 2024. PMID: 37857768
-
Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis.Sleep Med Rev. 2018 Aug;40:4-16. doi: 10.1016/j.smrv.2017.06.010. Epub 2017 Jul 6. Sleep Med Rev. 2018. PMID: 28890168
-
The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer's disease: a meta-analysis.Geroscience. 2025 Jun;47(3):3111-3130. doi: 10.1007/s11357-024-01488-3. Epub 2025 Jan 11. Geroscience. 2025. PMID: 39797935 Free PMC article.
-
Sleep, Cognitive impairment, and Alzheimer's disease: A Systematic Review and Meta-Analysis.Sleep. 2017 Jan 1;40(1). doi: 10.1093/sleep/zsw032. Sleep. 2017. PMID: 28364458
Cited by
-
The role of burnout prevention in promoting healthy aging: frameworks for the Semmelweis Study and Semmelweis-EUniWell Workplace Health Promotion Program.Geroscience. 2025 Jul 8. doi: 10.1007/s11357-025-01780-w. Online ahead of print. Geroscience. 2025. PMID: 40627122 Review.
-
Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications.Nutrients. 2025 Apr 15;17(8):1351. doi: 10.3390/nu17081351. Nutrients. 2025. PMID: 40284214 Free PMC article. Review.
References
-
- Chen J, Peng G, Sun B. Alzheimer’s disease and sleep disorders: a bidirectional relationship. Neuroscience. 2024;557:12–23. 10.1016/j.neuroscience.2024.08.008. - PubMed
-
- Shi L, Chen S-J, Ma M-Y, Bao Y-P, Han Y, Wang Y-M, Shi J, Vitiello MV, Lu L. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

