Viral Suppression, Viral Failure, and Safety Outcomes in Children and Adolescents With HIV on Dolutegravir in Europe and Thailand
- PMID: 40215206
- PMCID: PMC12596352
- DOI: 10.1093/cid/ciaf191
Viral Suppression, Viral Failure, and Safety Outcomes in Children and Adolescents With HIV on Dolutegravir in Europe and Thailand
Abstract
Background: Dolutegravir (DTG) is a preferred anchor antiretroviral therapy (ART) for children and adolescents with HIV (CAWH).
Methods: We assessed the effectiveness and safety of DTG in CAWH aged 0-18 years at DTG start in routine care in Europe and Thailand, evaluating viral suppression (viral load [VL] <50 copies/mL), cumulative incidence and associated factors of viral failure (VF; confirmed VL ≥400 copies/mL) and safety outcomes.
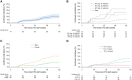
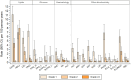
Results: Of 1230 CAWH on DTG, 49% were female. At DTG start, median (IQR) age was 14 (11-16) years, 10% were ART-naive, 49% ART-experienced/suppressed (VL <200 copies/mL), 13% ART-experienced/viremic (VL ≥200 copies/mL), and 28% ART-experienced/unknown VL. Median duration on DTG was 93 (49-163) weeks. Virall suppression was 88%-91% throughout follow-up. Cumulative incidence (95% CI) of VF at weeks 96 and 144 was 4.3% (3.1%-6.1%) and 8.3% (6.2%-11.1%). Increased risk of VF was associated with female sex, ART-experienced/viremic, advanced/severe immunosuppression, previous treatment failure, and region (P < .05, adjusting for age, sex and ART/VL status). The risk of VF was lower on DTG than CAWH on protease-inhibitor-based regimens (P < .001). Among 1146 with clinical data, 26 (2%) experienced 52 DTG-related adverse events, including 5 serious adverse events. Of 849 with laboratory data, 44 (5%) had 54 grade ≥3 events (<1 per 100 person-years). DTG discontinuation by weeks 96 and 144 was 5.0% (3.8%-6.7%) and 9.5% (7.5%-12.0%).
Conclusions: DTG was well tolerated, with ∼90% virally suppressed <50 copies/mL. VF was low overall but was significantly higher in children/adolescents ART-experienced and viraemic at DTG start, requiring close monitoring.
Keywords: ART; HIV; children/adolescents; dolutegravir; effectiveness.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. T. reports research support from the UK Research and Innovation MRC grant outside of the submitted work. A. T., E. C., C. F., C. J., M. M., and T. P. report support from ViiV Healthcare outside of the submitted work. H. C., I. J. C., L. C., C. F., T. G., S. C., A. J., C. J., M. M., L. M. P. T., and T. P. report support from Gilead Sciences outside of the submitted work. I. J. C. reports support from AbbVie outside of the submitted work. L. C. and C. J. report support from the European Union outside of the submitted work. L. C. reports support from European and Developing Countries Clinical Trials Partnership (EDCPT) and Janssen Pharmaceuticals outside of the submitted work. C. K. reports support from Bayer, Biotest, CSL Behring, Hämophilie Stiftung, Intersero, Novo Nordisk, Pfizer, Roche/Chugai, Takeda, Sobi/Sanofi, EU H2020 ITN, FUSE e.V., and State Hesse outside of the submitted work. A. N.-J. reports support from Biomérieux outside of the submitted work. A. B. and J. O. report support from the World Health Organization (WHO) outside of the submitted work. J. O. reports support from the United Nations Children's Fund (UNICEF) outside of the submitted work. C. H. and V. V. are employees of ViiV Healthcare, the sponsor of the research presented in the manuscript. As employees of ViiV Healthcare, C. H. and V. V. receive GlaxoSmithKline stocks. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. Author contributors . Writing Group (consisting of the lead authors, Project Team members, followed by Writing Group members (ordered alphabetically) and senior authors: lead authors and Project Team: Karen Scott, John O’Rourke, Charlotte Jackson, Luminita Ene, Luisa Galli, Tessa Goetghebuer, Cassidy Henegar, Christoph Königs, Magdalena Marczyńska, Lars Naver, Antoni Noguera-Julian, Paolo Paioni, Jose T. Ramos, Birgitte Smith, Wipaporn Natalie Songtaweesin, Vana Spoulou, Nattakarn Tantawarak, Anna Turkova, Vani Vannappagari, Alla Volokha, and Ali Judd; Writing Group members: Alastair Bamford, Hannah Castro, Elizabeth Chappell, Giorgia Dalla Valle, Caroline Foster, Sara Guillén Martín, Luis Manuel Prieto Tato, Thanyawee Puthanakit, Halyna Sherstiuk, Irina Shkurka, and Sandra Soeria-Atmadja; joint senior authors: Siobhan Crichton and Intira Jeannie Collins. The author contributors thank all collaborating partners. Declaration of interests . Cassidy Henegar and Vani Vannappagari are employees of ViiV Healthcare and receive GSK stock are part of their employment. EPPICC/Penta Coordinating Team. Elizabeth Chappell, Siobhan Crichton, Intira Jeannie Collins, Giorgia Dalla Valle, Charlotte Duff, Kate Edgar, Carlo Giaquinto, Charlotte Jackson, Ali Judd, Laura Mangiarini, John O’Rourke, Karen Scott, and Claire Thorne. Collaborating cohorts. Belgium: Hopital St Pierre Cohort, Brussels: Tessa Goetghebuer, MD, PhD; Marc Hainaut, MD PhD; Wivine Tremerie, Research nurse; Marc Delforge, data manager. Denmark: Copenhagen, Denmark: Thomas Ulrik Hoffmann, Sannie Brit Nordly, Birgitte Smith. Germany: Frankfurt, Germany: Christoph Königs. Greece: Greek cohort: Vana Spoulou. Italy: Infectious Disease Unit, Meyer Children's Hospital, IRCCS, Florence Italy; Department of Health Sciences, University of Florence, Florence, Italy: Luisa Galli, MD; Elena Chiappini, MD, PhD; Catiuscia Lisi, DStat; Carlotta Montagnani, MD PhD; Elisabetta Venturini, MD, PhD. Poland: Polish pediatric cohort: Head of the team: Prof Magdalena Marczyńska, MD, PhD. Members of the team: Jolanta Popielska, MD, PhD; Maria Pokorska-Śpiewak, MD, PhD; Agnieszka Ołdakowska, MD, PhD; Konrad Zawadka, MD, PhD; Magdalena Pluta MD, PhD. Administrative assistant: Małgorzata Doroba. Affiliation: Medical University of Warsaw, Poland, Department of Children's Infectious Diseases; Hospital of Infectious Diseases in Warsaw, Poland. Romania: “Victor Babes” Hospital Cohort, Bucharest: Dr Luminita Ene. Spain: CoRISPE-S and Madrid cohort: Received funding from Estudio del Análisis Clínico-Epidemiológico de la Infección por el Vih en Niños y Adolescentes, Mujeres Embarazadas y Sus Hijos a Nivel Nacional. Ministerio Sanidad; Project 202007PN0002. Pediatrics units: María José Mellado, Luis Escosa, Milagros García Hortelano, Talía Sainz, Carlos Grasa, Paula Rodríguez (Hospital Universitario La Paz, Madrid); Jose Tomás Ramos, Pablo Rojo, Luis Manuel Prieto Tato, Cristina Epalza, Alfredo Tagarro, Sara Domínguez, Álvaro Ballesteros (Hospital Universitario Doce de Octubre, Madrid); Marta Illán, Arantxa Berzosa, (Hospital Clínico San Carlos, Madrid); Sara Guillén, Beatriz Soto (Hospital Universitario de Getafe, Madrid); María Luisa Navarro, Jesús Saavedra, Mar Santos, Elena Rincón, David Aguilera, Begoña Santiago, Beatriz Lázaro Martín, Andrea López Suárez (Hospital Universitario Gregorio Marañón, Madrid); Amanda Bermejo (Hospital Universitario de Móstoles, Madrid); María Penín (Hospital Universitario Príncipe de Asturias de Alcalá de Henares, Madrid); Jorge Martínez (Hospital Infantil Universitario Niño Jesús, Madrid); Katie Badillo (Hospital Universitario de Torrejón, Madrid); Ana Belén Jiménez (Hospital Fundación Jiménez Díaz, Madrid); Adriana Navas (Hospital Universitario Infanta Leonor, Madrid); Eider Oñate (Hospital Universitario Donostia, Guipúzcoa); Itziar Pocheville (Hospital Universitario Cruces, Vizcaya); Elisa Garrote (Hospital Universitario Basurto, Vizcaya); Elena Colino, Olga Afonso (Hospital Insular Materno Infantil, Gran Canaria); Jorge Gómez Sirvent (Hospital Universitario Virgen de la Candelaria, Tenerife); Mónica Garzón, Vicente Román (Hospital General, Lanzarote); Raquel Angulo (Hospital de Poniente de El Ejido, Almería); Olaf Neth, Lola Falcón (Hospital Universitario Virgen del Rocío, Sevilla); Pedro Terol (Hospital Universitario Virgen de la Macarena, Sevilla); Juan Luis Santos, Álvaro Vázquez (Hospital Universitario Virgen de las Nieves, Granada); Begoña Carazo, Antonio Medina (Hospital Regional Universitario, Málaga); Francisco Lendínez, Mercedes Ibáñez (Complejo Hospitalario Torrecárdenas, Almería); Estrella Peromingo, María Isabel Sánchez (Hospital Universitario Puerta del Mar, Cádiz); Beatriz Ruiz (Hospital Universitario Reina Sofía de Córdoba); Ana Grande (Complejo Hospitalario Universitario Infanta Cristina, Badajoz); Francisco José Romero (Complejo Hospitalario, Cáceres); Carlos Pérez, Alejandra Méndez (Hospital de Cabueñes, Asturias); Laura Calle (Hospital Universitario Central de Asturias); Marta Pareja (Complejo Hospitalario Universitario, Albacete); Begoña Losada (Hospital Virgen de la Salud, Toledo); Mercedes Herranz (Hospital Virgen del Camino, Navarra); Matilde Bustillo (Hospital Universitario Miguel Servet, Zaragoza); Pilar Collado (Hospital Clínico Universitario Lozano Blesa, Zaragoza); José Antonio Couceiro (Complejo Hospitalario Universitario, Pontevedra); Leticia Vila (Complejo Hospitalario Universitario, La Coruña); Consuelo Calviño (Hospital Universitario Lucus Augusti, Lugo); Ana Isabel Piqueras, Manuel Oltra (Hospital Universitario La Fe, Valencia); César Gavilán (Hospital Universitario de San Juan de Alicante, Alicante); Elena Montesinos (Hospital General Universitario, Valencia); Marta Dapena (Hospital General, Castellón); Beatriz Jiménez (Hospital Universitario Marqués de Valdecilla, Cantabria); Ana Gloria Andrés (Complejo Hospitalario, León); Víctor Marugán, Carlos Ochoa (Complejo Hospitalario, Zamora); Ana Isabel Menasalvas, Eloísa Cervantes (Hospital Universitario Virgen de la Arrixaca, Murcia) and Pediatric HIV–BioBank integrated in the Spanish AIDS Research Network and collaborating centers. Adult units: Cristina Díez, (Hospital Universitario Gregorio Marañón, Madrid). Ignacio Bernardino, María Luisa Montes, Eulalia Valencia, Ana Delgado (Hospital Universitario La Paz, Madrid); Rafael Rubio, Federico Pulido, Otilia Bisbal (Hospital Universitario Doce de Octubre, Madrid); Alfonso Monereo Alonso (Hospital Universitario de Getafe, Madrid); Juan Berenguer, Cristina Díez, Teresa Aldamiz, Francisco Tejerina, Juan Carlos Bernaldo de Quirós, Belén Padilla, Raquel Carrillo, Pedro Montilla, Elena Bermúdez, Maricela Valerio (Hospital Universitario Gregorio Marañón, Madrid); Jose Sanz (Hospital Universitario Príncipe de Asturias de Alcalá de Henares, Madrid); Alejandra Gimeno (Hospital Universitario de Torrejon, Madrid); Miguel Cervero, Rafael Torres (Hospital Universitario Severo Ochoa de Leganés, Madrid); Santiago Moreno, María Jesús Perez, Santos del Campo (Hospital Universitario Ramon y Cajal, Madrid); Pablo Ryan, Jesus Troya (Hospital Universitario Infanta Leonor, Madrid); Jesus Sanz (Hospital Universitario La Princesa, Madrid); Juan Losa, Rafael Gomez (Hospital Universitario Fundacion Alcorcon, Madrid); Miguel Górgolas (Hospital Fundacion Jimenez Diaz, Madrid); Alberto Díaz, Sara de la Fuente (Hospital Universitario Puerta de Hierro de Majadahonda, Madrid); Jose Antonio Iribarren, Marıa Jose Aramburu, Lourdes Martinez (Hospital Universitario Donostia, Guipuzcoa); Ane Josune Goikoetxea (Hospital Universitario Cruces, Vizcaya); Sofia Ibarra, Mireia de la Peña (Hospital Universitario Basurto, Vizcaya); Víctor Asensi (Hospital Universitario Central de Asturias); Michele Hernandez (Hospital Universitario Insular, Gran Canaria); María Remedios Alemán, Ricardo Pelazas, María del Mar Alonso, Ana María López, Dácil García, Jehovana Rodriguez (Hospital Universitario de Canarias, Tenerife); Miguel Angel Cardenes (Hospital Universitario Doctor Negrin, Gran Canaria); Manuel A. Castaño, Francisco Orihuela, Inés Pérez, Mª Isabel Mayorga (Hospital Regional Universitario, Málaga); Luis Fernando Lopez-Cortes, Cristina Roca, Silvia Llaves (Hospital Universitario Virgen del Rocio, Sevilla); Marıa Jose Rios, Jesus Rodriguez, Virginia Palomo (Hospital Universitario Virgen de la Macarena, Sevilla); Juan Pasquau, Coral Garcia (Hospital Universitario Virgen de las Nieves, Granada); Jose Hernandez, Clara Martinez (Hospital Universitario Clinico San Cecilio, Granada); Antonio Rivero, Angela Camacho (Hospital Universitario Reina Sofia, Cordoba); Dolores Merino, Miguel Raffo, Laura Corpa (Hospital Universitario Juan Ramon Jimenez, Huelva); Elisa Martinez, Fernando Mateos, Jose Javier Blanch (Complejo Hospitalario Universitario, Albacete); Miguel Torralba (Hospital Universitario, Guadalajara); Piedad Arazo, Gloria Samperiz (Hospital Universitario Miguel Servet, Zaragoza); Celia Miralles, Antonio Ocampo, Guille Pousada (Hospital Alvaro Cunqueiro, Pontevedra); Alvaro Mena (Complejo Hospitalario Universitario, La Coruna); Marta Montero, Miguel Salavert, (Hospital Universitario La Fe, Valencia); Maria Jose Galindo, Natalia Pretel (Hospital Clinico Universitario, Valencia); Joaquín Portilla, Irene Portilla (Hospital General Universitario, Alicante); Felix Gutierrez, Mar Masia, Cati Robledano, Araceli Adsuar (Hospital General Universitario de Elche, Alicante); Carmen Hinojosa, Begoña Monteagudo (Hospital Clinico, Valladolid); Pablo Bachiller (Hospital General, Segovia); Jesica Abadía (Hospital Universitario Rio Hortega, Valladolid); Carlos Galera, Helena Albendin, Marian Fernandez (Hospital Universitario Virgen de la Arrixaca, Murcia); Jose Ramon Blanco (Complejo Hospitalario San Millan-San Pedro, la Rioja). Spain: CoRISPE-cat, Catalonia: CoRISPE-cat receives financial support from the Instituto de Salud Carlos III through the Red Temática de Investigación Cooperativa en Sida (grant numbers RED RIS RD06/0006/0035 yRD06/0006/0021). Members: Hospital Universitari Vall d’Hebron, Barcelona (Pere Soler-Palacín, Beatriz Álvarezand Santiago Pérez-Hoyos [statistician]), Hospital Universitari del Mar, Barcelona (Núria López), Hospital Universitari Germans Trias i Pujol, Badalona (María Méndez, Clara Carreras), Hospital Universitari JosepTrueta, Girona (Borja Guarch), Hospital Universitari Arnau de Vilanova, Lleida (Teresa Vallmanya, Laura Minguell-Domingo), Hospital Universitari Joan XXIII, Tarragona (Olga Calavia), Consorci Sanitari del Maresme, Mataró (Lourdes García), Hospital General de Granollers (Maite Coll, Berta Pujol), Corporació Sanitària Parc Taulí, Sabadell (Valentí Pineda), Hospital Universitari Sant Joan, Reus (Neus Rius), Fundació Althaia, Manresa (Núria Rovira), Hospital Son Espases, Mallorca (Joaquín Dueñas) and Hospital Sant Joan de Déu, Esplugues (Clàudia Fortuny, Anna Gamell, Antoni Noguera-Julian). Sweden: Karolinska University Hospital, Stockholm, The Swedish InfCareHIV cohort (Lars Navér, Sandra Soeria-Atmadja, Vendela Hagås, Johanna Rubin, Nora Einarsson). Switzerland: Members of the Swiss HIV Cohort Study (SHCS) and the Swiss Mother and Child HIV Cohort (MoCHiV) Study: I. A. Abela, K. Aebi-Popp, A. Anagnostopoulos, M. Battegay, M. Baumann, E. Bernasconi, D. L. Braun, H. C. Bucher, A. Calmy, M. Cavassini (Chairman of the Clinical and Laboratory Committee), A. Ciuffi, P. A. Crisinel, K. E. A. Darling, G. Dollenmaier, A. Duppenthaler, M. Egger, L. Elzi, J. S. Fehr, J. Fellay, K. Francini, H. Furrer, C. A. Fux, H. F. Günthard (President of the SHCS), A. Hachfeld, D. H. U. Haerry (deputy of “Positive Council”), B. Hasse, H. H. Hirsch, M. Hoffmann, I. Hösli, M. Huber, D. Jackson-Perry (patient representatives), C. R. Kahlert (Chairman of the Mother and Child Substudy), O. Keiser, T. Klimkait, M. Kohns, L. Kottanattu, R. D. Kouyos, H. Kovari, K. Kusejko (Head of Data Center), N. D. Labhardt, Karoline Leuzinger, B. Martinez de Tejada, C. Marzolini, K. J. Metzner, N. Müller, J. Nemeth, D. Nicca, J. Notter, P. Paioni, G. Pantaleo, M. Perreau, Ch. Polli, E. Ranieri, A. Rauch, L. P. Salazar-Vizcaya, P. Schmid, O. Segeral, R. F. Speck, M. Stöckle, P. E. Tarr, M. Thanh Lecompte, A. Trkola, N. Wagner, G. Wandeler (Chairman of the Scientific Board), M. Weisser, S. Yerly. This study was financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant number 201369). Thailand: Center of Excellence for Pediatric Infectious Diseases and Vaccines, Faculty of Medicine, Chulalongkorn University: Rachaneekorn Nadsasarn, Chutima Saisaengjan, Patama Deeklum, Phattharapa Khamkhen, Lucksanapon Pitikawinwong. Infectious Disease Unit, Department of Pediatrics, Faculty of Medicine, Khon Kaen University: Nattakarn Tantawarak, MD, Pope Kosalaraksa, MD, Chanasda Kakkaew, Piangjit Tharnprisan. United Kingdom/Ireland: Collaborative HIV Paediatric Study (CHIPS): CHIPS was funded by the NHS (London Specialized Commissioning Group) and received additional support from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, Janssen, and Roche. The MRC Clinical Trials Unit at UCL is supported by the Medical Research Council (https://www.mrc.ac.uk) program number MC_UU_00004/03. CHIPS Steering Committee: Hermione Lyall (chair), Alasdair Bamford, Karina Butler, Katja Doerholt, Conor Doherty, Caroline Foster, Ian Harrison, Julia Kenny, Nigel Klein, Gillian Letting, Paddy McMaster, Fungai Murau, Edith Nsangi, Katia Prime, Andrew Riordan, Fiona Shackley, Delane Shingadia, Sharon Storey, Gareth Tudor-Williams, Anna Turkova, Steve Welch. MRC Clinical Trials Unit: Intira Jeannie Collins, Claire Cook, Siobhan Crichton, Donna Dobson, Keith Fairbrother, Diana M. Gibb, Ali Judd, Marthe Le Prevost, Nadine Van Looy. Integrated Screening Outcome Surveillance Service (ISOSS), UCL: Helen Peters, Kate Francis, Claire Thorne. Hospitals participating in CHIPS in 2019/2020: University Hospitals Birmingham NHS Foundation Trust, Birmingham: L. Thrasyvoulou, S. Welch; Brighton and Sussex University Hospitals NHS Trust: K. Fidler; University Hospitals Bristol NHS Foundation Trust, Bristol: J. Bernatoniene, F. Manyika; Calderdale and Huddersfield NHS Foundation Trust, Halifax: G. Sharpe; Derby Teaching Hospitals NHS Foundation Trust: B. Subramaniam; Glasgow Royal Hospital for Children, Glasgow: R. Hague, V. Price; Great Ormond Street Hospital for Children NHS Foundation Trust, London: J. Flynn, N. Klein, A. Bamford, D. Shingadia, K. Grant; Oxford University Hospitals NHS Foundation Trust, Oxford: S. Yeadon, S. Segal; King's College Hospital NHS Foundation Trust, London: S. Hawkins; Leeds Teaching Hospitals NHS Trust, Leeds: M. Dowie; University Hospitals of Leicester NHS Trust, Leicester: S. Bandi, E. Percival; Luton and Dunstable Hospital NHS Foundation Trust, Luton: M. Eisenhut; K. Duncan; Milton Keynes General University Hospital NHS Foundation Trust, Milton Keynes: L. Anguvaa, L. Wren, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle: T. Flood, A. Pickering; The Pennine Acute Hospitals NHS Trust, Manchester: P. McMaster, C. Murphy; North Middlesex University Hospital NHS Trust, London: J. Daniels, Y. Lees; Northampton General Hospital NHS Trust, Northampton: F. Thompson; London North West Healthcare NHS Trust, Middlesex: A. Williams, B. Williams, S. Pope; Barts Health NHS Trust, London: Dr S. Libeschutz; Nottingham University Hospitals NHS Trust, Nottingham: L. Cliffe, S. Southall; Portsmouth Hospitals NHS Trust, Portsmouth: A. Freeman; Raigmore Hospital, Inverness: H. Freeman; Royal Belfast Hospital for Sick Children, Belfast: S. Christie; Royal Berkshire NHS Foundation Trust, Reading: A. Gordon; Royal Children's Hospital, Aberdeen: D. Rosie Hague, L. Clarke; Royal Edinburgh Hospital for Sick Children, Edinburgh: L. Jones, L. Brown; Royal Free NHS Foundation Trust, London: M. Greenberg; Alder Hey Children's NHS Foundation Trust, Liverpool: C. Benson, A. Riordan; Sheffield Children's NHS Foundation Trust, Sheffield: L. Ibberson, F. Shackley; University Hospital Southampton NHS Foundation Trust, Southampton: S. Patel, J. Hancock; St George's University Hospitals NHS Foundation Trust, London: K. Doerholt, K. Prime, M. Sharland, S. Storey; Imperial College Healthcare NHS Trust, London: E. G. H. Lyall, C. Foster, P. Seery, G. Tudor-Williams, N. Kirkhope, S. Raghunanan; Guy's and St Thomas’ NHS Foundation Trust, London: Dr Julia Kenny, A. Callaghan; University Hospitals of North Midlands NHS Trust, Stoke on Trent: A. Bridgwood, P. McMaster; University Hospital of Wales, Cardiff: J. Evans, E. Blake; NHS Frimley Health Foundation Trust, Slough: A. Yannoulias. Ukraine: Pediatric HIV Cohort: Dr T. Kaleeva, Dr Y. Baryshnikova (Odessa Regional Centre for HIV/AIDS); Dr S. Soloha (Donetsk Regional Centre for HIV/AIDS); Dr N. Bashkatova (Mariupol AIDS Center); Dr I. Raus (Kiev City Centre for HIV/AIDS); Dr O. Glutshenko, Dr Z. Ruban (Mykolaiv Regional Centre for HIV/AIDS); Dr N. Prymak (Kryvyi Rih); Dr G. Kiseleva (Simferopol); Dr Alla Volokha (Shupyk National Medical Academy of Postgraduate Education); Dr Ruslan Malyuta (Perinatal Prevention of AIDS Initiative, Odessa); Dr H. Bailey, Prof Claire Thorne (UCL, London, United Kingdom). Funding acknowledgment: PENTA Foundation. Data sharing statement . The EPPICC data are held at MRC Clinical Trials Unit at UCL, which encourages optimal use of data by employing a controlled access approach to data sharing, incorporating a transparent and robust system to review requests and provide secure data access consistent with the relevant ethics committee approvals. The rationale for this approach has been published (doi: 10.1186/s13063–015-0604-6). Ethics committee approval for use of EPPICC data restricts the ability for EPPICC data to be shared publicly without request. Rather, ethics approval does allow a controlled access approach. All requests for data are considered and can be initiated by contacting mrcctu.datareleaserequest@ucl.ac.uk.
Figures


References
-
- World Health Organization (WHO) . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. July 2021. Available at: https://www.who.int/publications-detail-redirect/9789240031593. Accessed 25 July 2022. - PubMed
-
- Blanco-Arévalo JL, García-Deltoro M, Torralba M, et al. HIV-1 resistance and virological failure to treatment with the integrase inhibitors bictegravir, cabotegravir, and dolutegravir: a systematic literature review. AIDS Rev 2024; 26:67–79. - PubMed
-
- Peñafiel J, de Lazzari E, Padilla M, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother 2017; 72:1752–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

